Loading of Indocyanine Green within Polydopamine-Coated Laponite Nanodisks for Targeted Cancer Photothermal and Photodynamic Therapy
- PMID: 29783745
- PMCID: PMC5977361
- DOI: 10.3390/nano8050347
Loading of Indocyanine Green within Polydopamine-Coated Laponite Nanodisks for Targeted Cancer Photothermal and Photodynamic Therapy
Abstract
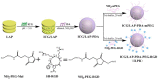
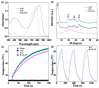
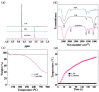
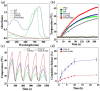
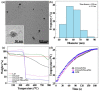
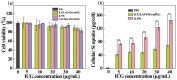
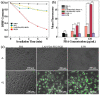
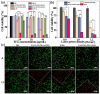
The combination of photothermal therapy (PTT) and photodynamic therapy (PDT) in cancer treatment has attracted much attention in recent years. However, developing highly efficient and targeted therapeutic nanoagents for amplifying PTT and PDT treatments remains challenging. In this work, we developed a novel photothermal and photodynamic therapeutic nanoplatform for treatment of cancer cells overexpressing integrin αvβ₃ through the coating of polydopamine (PDA) on indocyanine green (ICG)-loaded laponite (LAP) and then further conjugating polyethylene glycol-arginine-glycine-aspartic acid (PEG-RGD) as targeted agents on the surface. The ICG/LAP⁻PDA⁻PEG⁻RGD (ILPR) nanoparticles (NPs) formed could load ICG with a high encapsulation efficiency of 94.1%, improve the photostability of loaded ICG dramatically via the protection of PDA and LAP, and display excellent colloidal stability and biocompatibility due to the PEGylation. Under near-infrared (NIR) laser irradiation, the ILPR NPs could exert enhanced photothermal conversion reproducibly and generate reactive oxygen species (ROS) efficiently. More importantly, in vitro experiments proved that ILPR NPs could specifically target cancer cells overexpressing integrin αvβ₃, enhance cellular uptake due to RGD-mediated targeting, and exert improved photothermal and photodynamic killing efficiency against targeted cells under NIR laser irradiation. Therefore, ILPR may be used as effective therapeutic nanoagents with enhanced photothermal conversion performance and ROS generating ability for targeted PTT and PDT treatment of cancer cells with integrin αvβ₃ overexpressed.
Keywords: indocyanine green; laponite; photodynamic therapy; photothermal therapy; polydopamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Song S., He S., Tao Y., Wang L., Han F., Chen H., Zhang Z. Indocyanine green loaded magnetic carbon nanoparticles for near infrared fluorescence/magnetic resonance dual-modal imaging and photothermal therapy of tumor. ACS Appl. Mater. Interfaces. 2017;9:9484–9495. doi: 10.1021/acsami.7b00490. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

