Erythrocyte microRNA sequencing reveals differential expression in relapsing-remitting multiple sclerosis
- PMID: 29783973
- PMCID: PMC5963124
- DOI: 10.1186/s12920-018-0365-7
Erythrocyte microRNA sequencing reveals differential expression in relapsing-remitting multiple sclerosis
Abstract
Background: There is a paucity of knowledge concerning erythrocytes in the aetiology of Multiple Sclerosis (MS) despite their potential to contribute to disease through impaired antioxidant capacity and altered haemorheological features. Several studies have identified an abundance of erythrocyte miRNAs and variable profiles associated with disease states, such as sickle cell disease and malaria. The aim of this study was to compare the erythrocyte miRNA profile of relapsing-remitting MS (RRMS) patients to healthy sex- and age-matched controls.
Methods: Erythrocytes were purified by density-gradient centrifugation and RNA was extracted. Following library preparation, samples were run on a HiSeq4000 Illumina instrument (paired-end 100 bp sequencing). Sequenced erythrocyte miRNA profiles (9 patients and 9 controls) were analysed by DESeq2. Differentially expressed miRNAs were validated by RT-qPCR using miR-152-3p as an endogenous control and replicated in a larger cohort (20 patients and 18 controls). After logarithmic transformation, differential expression was determined by two-tailed unpaired t-tests. Logistic regression analysis was carried out and receiver operating characteristic (ROC) curves were generated to determine biomarker potential.
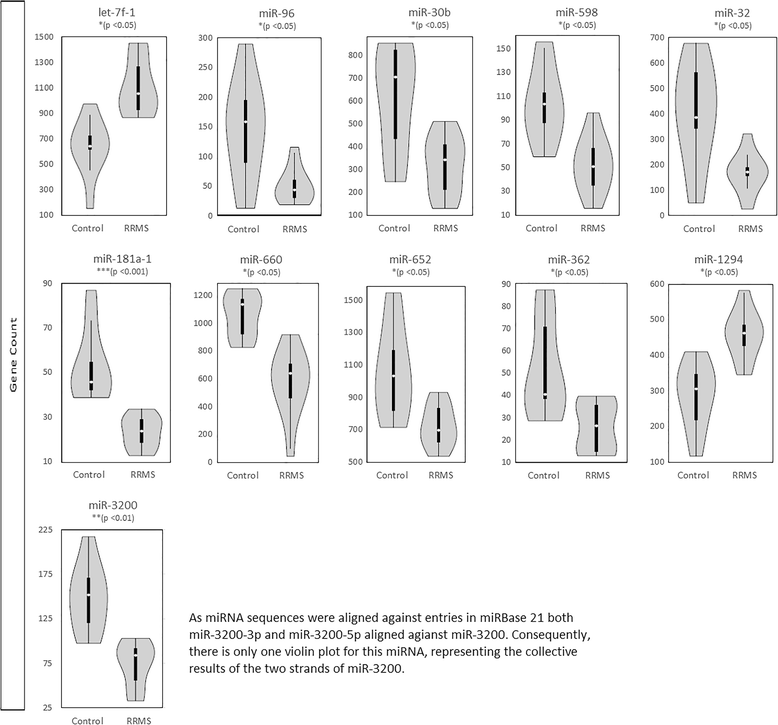
Results: A total of 236 erythrocyte miRNAs were identified. Of twelve differentially expressed miRNAs in RRMS two showed increased expression (adj. p < 0.05). Only modest fold-changes were evident across differentially expressed miRNAs. RT-qPCR confirmed differential expression of miR-30b-5p (0.61 fold, p < 0.05) and miR-3200-3p (0.36 fold, p < 0.01) in RRMS compared to healthy controls. Relative expression of miR-3200-5p (0.66 fold, NS p = 0.096) also approached significance. MiR-3200-5p was positively correlated with cognition measured by audio-recorded cognitive screen (r = 0.60; p < 0.01). MiR-3200-3p showed greatest biomarker potential as a single miRNA (accuracy = 75.5%, p < 0.01, sensitivity = 72.7%, specificity = 84.0%). Combining miR-3200-3p, miR-3200-5p, and miR-30b-5p into a composite biomarker increased accuracy to 83.0% (p < 0.05), sensitivity to 77.3%, and specificity to 88.0%.
Conclusions: This is the first study to report differences in erythrocyte miRNAs in RRMS. While the role of miRNAs in erythrocytes remains to be elucidated, differential expression of erythrocyte miRNAs may be exploited as biomarkers and their potential contribution to MS pathology and cognition should be further investigated.
Keywords: Erythrocytes; Next-generation sequencing; Relapsing-remitting multiple sclerosis; microRNA.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was obtained from the Bond University Human Research Ethics Committee (RO-1382), the University of Newcastle Ethics Committee (H-505-0607), and the Hunter Area Research Ethics Committee (no. 05/04/13/3.09). All participants gave written informed consent prior to enrolment.
Competing interests
JLS’s institution receives non-directed funding, as well as honoraria for presentations and membership on advisory boards from Sanofi Aventis, Biogen Idec, Bayer Health Care, Merck Serono, Teva, Roche, and Novartis Australia.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Exosomal microRNA signatures in multiple sclerosis reflect disease status.Sci Rep. 2017 Oct 30;7(1):14293. doi: 10.1038/s41598-017-14301-3. Sci Rep. 2017. PMID: 29084979 Free PMC article.
-
Next-generation sequencing reveals broad down-regulation of microRNAs in secondary progressive multiple sclerosis CD4+ T cells.Clin Epigenetics. 2016 Aug 27;8(1):87. doi: 10.1186/s13148-016-0253-y. eCollection 2016. Clin Epigenetics. 2016. PMID: 27570566 Free PMC article.
-
Downregulation of miR-125a-5p and miR-218-5p in Peripheral Blood Mononuclear Cells of Patients with Relapsing-Remitting Multiple Sclerosis.Immunol Invest. 2022 Jul;51(5):1149-1161. doi: 10.1080/08820139.2021.1909616. Epub 2021 Apr 19. Immunol Invest. 2022. PMID: 33866949
-
Serum Based miRNA as a Diagnostic Biomarker for Multiple Sclerosis: a Systematic Review and Meta-Analysis.Immunol Invest. 2022 May;51(4):947-962. doi: 10.1080/08820139.2021.1887888. Epub 2021 Mar 4. Immunol Invest. 2022. PMID: 33660581
-
Expression, regulation and function of microRNAs in multiple sclerosis.Int J Med Sci. 2014 Jun 2;11(8):810-8. doi: 10.7150/ijms.8647. eCollection 2014. Int J Med Sci. 2014. PMID: 24936144 Free PMC article. Review.
Cited by
-
Bioinformatics Analysis of Next Generation Sequencing Data Identifies Molecular Biomarkers Associated With Type 2 Diabetes Mellitus.Clin Med Insights Endocrinol Diabetes. 2023 Feb 20;16:11795514231155635. doi: 10.1177/11795514231155635. eCollection 2023. Clin Med Insights Endocrinol Diabetes. 2023. PMID: 36844983 Free PMC article.
-
Emerging concepts in the molecular cell biology and functions of mammalian erythrocytes.J Biol Chem. 2025 Apr;301(4):108331. doi: 10.1016/j.jbc.2025.108331. Epub 2025 Feb 19. J Biol Chem. 2025. PMID: 39984047 Free PMC article. Review.
-
MicroRNA-3200-3p targeting CAMK2A modulates the proliferation and metastasis of glioma in vitro.Bioengineered. 2022 Mar;13(3):7785-7797. doi: 10.1080/21655979.2022.2048995. Bioengineered. 2022. PMID: 35287547 Free PMC article.
-
miRNA contributions to pediatric-onset multiple sclerosis inferred from GWAS.Ann Clin Transl Neurol. 2019 May 15;6(6):1053-1061. doi: 10.1002/acn3.786. eCollection 2019 Jun. Ann Clin Transl Neurol. 2019. PMID: 31211169 Free PMC article.
-
Epstein-Barr Virus and miRNAs: Partners in Crime in the Pathogenesis of Multiple Sclerosis?Front Immunol. 2019 Apr 3;10:695. doi: 10.3389/fimmu.2019.00695. eCollection 2019. Front Immunol. 2019. PMID: 31001286 Free PMC article. Review.
References
-
- Marieb EN, Hoehn K. Human anatomy & physiology. Boston: Pearson; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

