Epigenetic switch from repressive to permissive chromatin in response to cold stress
- PMID: 29784800
- PMCID: PMC6003311
- DOI: 10.1073/pnas.1721241115
Epigenetic switch from repressive to permissive chromatin in response to cold stress
Abstract
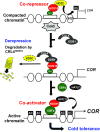
Switching from repressed to active status in chromatin regulation is part of the critical responses that plants deploy to survive in an ever-changing environment. We previously reported that HOS15, a WD40-repeat protein, is involved in histone deacetylation and cold tolerance in Arabidopsis However, it remained unknown how HOS15 regulates cold responsive genes to affect cold tolerance. Here, we show that HOS15 interacts with histone deacetylase 2C (HD2C) and both proteins together associate with the promoters of cold-responsive COR genes, COR15A and COR47 Cold induced HD2C degradation is mediated by the CULLIN4 (CUL4)-based E3 ubiquitin ligase complex in which HOS15 acts as a substrate receptor. Interference with the association of HD2C and the COR gene promoters by HOS15 correlates with increased acetylation levels of histone H3. HOS15 also interacts with CBF transcription factors to modulate cold-induced binding to the COR gene promoters. Our results here demonstrate that cold induces HOS15-mediated chromatin modifications by degrading HD2C. This switches the chromatin structure status and facilitates recruitment of CBFs to the COR gene promoters. This is an apparent requirement to acquire cold tolerance.
Keywords: CUL4-based E3 ligase; HOS15; cold stress response; derepression; histone acetylation.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Jeon J, Kim J. Cold stress signaling networks in Arabidopsis. J Plant Biol. 2013;56:69–76.
-
- Chinnusamy V, Zhu J, Zhu J-K. Gene regulation during cold acclimation in plants. Physiol Plant. 2006;126:52–61.
-
- Thomashow MF. PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. - PubMed
-
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: An overview. Arch Biochem Biophys. 2005;444:139–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

