Continuous intrathecal orexin delivery inhibits cataplexy in a murine model of narcolepsy
- PMID: 29784823
- PMCID: PMC6003319
- DOI: 10.1073/pnas.1722686115
Continuous intrathecal orexin delivery inhibits cataplexy in a murine model of narcolepsy
Abstract
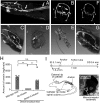
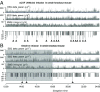
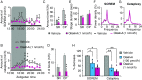
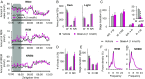
Narcolepsy-cataplexy is a chronic neurological disorder caused by loss of orexin (hypocretin)-producing neurons, associated with excessive daytime sleepiness, sleep attacks, cataplexy, sleep paralysis, hypnagogic hallucinations, and fragmentation of nighttime sleep. Currently, human narcolepsy is treated by providing symptomatic therapies, which can be associated with an array of side effects. Although peripherally administered orexin does not efficiently penetrate the blood-brain barrier, centrally delivered orexin can effectively alleviate narcoleptic symptoms in animal models. Chronic intrathecal drug infusion through an implantable pump is a clinically available strategy to treat a number of neurological diseases. Here we demonstrate that the narcoleptic symptoms of orexin knockout mice can be reversed by lumbar-level intrathecal orexin delivery. Orexin was delivered via a chronically implanted intrathecal catheter at the upper lumbar level. The computed tomographic scan confirmed that intrathecally administered contrast agent rapidly moved from the spinal cord to the brain. Intrathecally delivered orexin was detected in the brain by radioimmunoassay at levels comparable to endogenous orexin levels. Cataplexy and sleep-onset REM sleep were significantly decreased in orexin knockout mice during and long after slow infusion of orexin (1 nmol/1 µL/h). Sleep/wake states remained unchanged both quantitatively as well as qualitatively. Intrathecal orexin failed to induce any changes in double orexin receptor-1 and -2 knockout mice. This study supports the concept of intrathecal orexin delivery as a potential therapy for narcolepsy-cataplexy to improve the well-being of patients.
Keywords: lumbar spinal cord; non-REM sleep; orexin knockout mice; slow infusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models.Proc Natl Acad Sci U S A. 2017 May 30;114(22):5731-5736. doi: 10.1073/pnas.1700499114. Epub 2017 May 15. Proc Natl Acad Sci U S A. 2017. PMID: 28507129 Free PMC article.
-
TAK-994, a Novel Orally Available Brain-Penetrant Orexin 2 Receptor-Selective Agonist, Suppresses Fragmentation of Wakefulness and Cataplexy-Like Episodes in Mouse Models of Narcolepsy.J Pharmacol Exp Ther. 2023 Jun;385(3):193-204. doi: 10.1124/jpet.122.001449. Epub 2023 Mar 31. J Pharmacol Exp Ther. 2023. PMID: 37001988
-
OX2R-selective orexin agonism is sufficient to ameliorate cataplexy and sleep/wake fragmentation without inducing drug-seeking behavior in mouse model of narcolepsy.PLoS One. 2022 Jul 22;17(7):e0271901. doi: 10.1371/journal.pone.0271901. eCollection 2022. PLoS One. 2022. PMID: 35867683 Free PMC article.
-
Narcolepsy in the older adult: epidemiology, diagnosis and management.Drugs Aging. 2003;20(5):361-76. doi: 10.2165/00002512-200320050-00005. Drugs Aging. 2003. PMID: 12696996 Review.
-
Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system.Sleep Med Rev. 2005 Aug;9(4):269-310. doi: 10.1016/j.smrv.2005.03.004. Sleep Med Rev. 2005. PMID: 16006155 Review.
Cited by
-
A Century Searching for the Neurons Necessary for Wakefulness.Front Neurosci. 2022 Jul 19;16:930514. doi: 10.3389/fnins.2022.930514. eCollection 2022. Front Neurosci. 2022. PMID: 35928009 Free PMC article. Review.
-
Narcolepsy and rapid eye movement sleep.J Sleep Res. 2025 Apr;34(2):e14277. doi: 10.1111/jsr.14277. Epub 2024 Jul 2. J Sleep Res. 2025. PMID: 38955433 Free PMC article. Review.
-
Role of spontaneous and sensory orexin network dynamics in rapid locomotion initiation.Prog Neurobiol. 2020 Apr;187:101771. doi: 10.1016/j.pneurobio.2020.101771. Epub 2020 Feb 11. Prog Neurobiol. 2020. PMID: 32058043 Free PMC article.
-
Hypocretin/Orexin Receptor Pharmacology and Sleep Phases.Front Neurol Neurosci. 2021;45:22-37. doi: 10.1159/000514963. Epub 2021 May 28. Front Neurol Neurosci. 2021. PMID: 34052813 Free PMC article. Review.
-
Hypocretin (Orexin) Replacement Therapies.Med Drug Discov. 2020 Dec;8:100070. doi: 10.1016/j.medidd.2020.100070. Epub 2020 Oct 17. Med Drug Discov. 2020. PMID: 38738170 Free PMC article.
References
-
- Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. - PubMed
-
- Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. - PubMed
-
- Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. - PubMed
-
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

