Oxidative Stress and First-Line Antituberculosis Drug-Induced Hepatotoxicity
- PMID: 29784840
- PMCID: PMC6105810
- DOI: 10.1128/AAC.02637-17
Oxidative Stress and First-Line Antituberculosis Drug-Induced Hepatotoxicity
Erratum in
-
Erratum for Yew et al., "Oxidative Stress and First-Line Antituberculosis Drug-Induced Hepatotoxicity".Antimicrob Agents Chemother. 2018 Sep 24;62(10):e01633-18. doi: 10.1128/AAC.01633-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30249717 Free PMC article. No abstract available.
Abstract
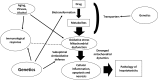
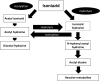
Hepatotoxicity induced by antituberculosis drugs is a serious adverse reaction with significant morbidity and even, rarely, mortality. This form of toxicity potentially impacts the treatment outcome of tuberculosis in some patients. Covering only first-line antituberculosis drugs, this review addresses whether and how oxidative stress and, more broadly, disturbance in redox homeostasis alongside mitochondrial dysfunction may contribute to the hepatotoxicity induced by them. Risk factors for such toxicity that have been identified, in addition to genetic factors, principally include old age, malnutrition, alcoholism, chronic hepatitis C and chronic hepatitis B infection, HIV infection, and preexisting liver disease. Importantly, these comorbid conditions are associated with oxidative stress. Thus, the shared pathogenetic mechanism(s) for liver injury might be in operation due to disease-drug interaction. Our current ability to predict, prevent, or treat hepatotoxicity (other than removing potentially hepatotoxic drugs) remains limited. More translational research to unravel the pathogenesis, inclusive of the underlying molecular basis, regarding antituberculosis drug-induced hepatotoxicity is needed, and so is clinical research pertaining to the advances in therapy with antioxidants and drugs related to antioxidants, especially those for management of mitochondrial dysfunction. The role of pharmacogenetics in the clinical management of drug-induced hepatotoxicity also likely merits further evaluation.
Keywords: drugs; hepatotoxicity; tuberculosis.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR, ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee . 2006. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 174:935–952. doi: 10.1164/rccm.200510-1666ST. - DOI - PubMed
-
- Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O'Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. 2016. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 63:e147–e195. doi: 10.1093/cid/ciw376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

