A human-specific, truncated α7 nicotinic receptor subunit assembles with full-length α7 and forms functional receptors with different stoichiometries
- PMID: 29784875
- PMCID: PMC6036215
- DOI: 10.1074/jbc.RA117.001698
A human-specific, truncated α7 nicotinic receptor subunit assembles with full-length α7 and forms functional receptors with different stoichiometries
Abstract
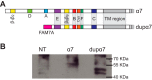
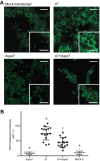
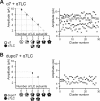
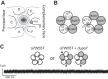
The cholinergic α7 nicotinic receptor gene, CHRNA7, encodes a subunit that forms the homopentameric α7 receptor, involved in learning and memory. In humans, exons 5-10 in CHRNA7 are duplicated and fused to the FAM7A genetic element, giving rise to the hybrid gene CHRFAM7A Its product, dupα7, is a truncated subunit lacking part of the N-terminal extracellular ligand-binding domain and is associated with neurological disorders, including schizophrenia, and immunomodulation. We combined dupα7 expression on mammalian cells with patch clamp recordings to understand its functional role. Transfected cells expressed dupα7 protein, but they exhibited neither surface binding of the α7 antagonist α-bungarotoxin nor responses to acetylcholine (ACh) or to an allosteric agonist that binds to the conserved transmembrane region. To determine whether dupα7 assembles with α7, we generated receptors comprising α7 and dupα7 subunits, one of which was tagged with conductance substitutions that report subunit stoichiometry and monitored ACh-elicited channel openings in the presence of a positive allosteric α7 modulator. We found that α7 and dupα7 subunits co-assemble into functional heteromeric receptors, which require at least two α7 subunits for channel opening, and that dupα7's presence in the pentameric arrangement does not affect the duration of the potentiated events compared with that of α7. Using an α7 subunit mutant, we found that activation of (α7)2(dupα7)3 receptors occurs through ACh binding at the α7/α7 interfacial binding site. Our study contributes to the understanding of the modulation of α7 function by the human specific, duplicated subunit, associated with human disorders.
Keywords: Cys-loop receptor; channel activation; ion channel; nicotinic acetylcholine receptors (nAChR); patch clamp; receptor structure-function; single-channel recording; α7 receptor.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Sauguet L., Shahsavar A., Poitevin F., Huon C., Menny A., Nemecz À., Haouz A., Changeux J.-P., Corringer P.-J., and Delarue M. (2014) Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc. Natl. Acad. Sci. U.S.A. 111, 966–971 10.1073/pnas.1314997111 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

