Adapting to oxygen: 3-Hydroxyanthrinilate 3,4-dioxygenase employs loop dynamics to accommodate two substrates with disparate polarities
- PMID: 29784877
- PMCID: PMC6036192
- DOI: 10.1074/jbc.RA118.002698
Adapting to oxygen: 3-Hydroxyanthrinilate 3,4-dioxygenase employs loop dynamics to accommodate two substrates with disparate polarities
Abstract
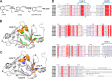
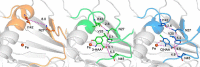
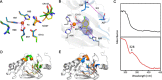
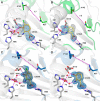
3-Hydroxyanthranilate 3,4-dioxygenase (HAO) is an iron-dependent protein that activates O2 and inserts both oxygen atoms into 3-hydroxyanthranilate (3-HAA). An intriguing question is how HAO can rapidly bind O2, even though local O2 concentrations and diffusion rates are relatively low. Here, a close inspection of the HAO structures revealed that substrate- and inhibitor-bound structures exhibit a closed conformation with three hydrophobic loop regions moving toward the catalytic iron center, whereas the ligand-free structure is open. We hypothesized that these loop movements enhance O2 binding to the binary complex of HAO and 3-HAA. We found that the carboxyl end of 3-HAA triggers changes in two loop regions and that the third loop movement appears to be driven by an H-bond interaction between Asn27 and Ile142 Mutational analyses revealed that N27A, I142A, and I142P variants cannot form a closed conformation, and steady-state kinetic assays indicated that these variants have a substantially higher Km for O2 than WT HAO. This observation suggested enhanced hydrophobicity at the iron center resulting from the concerted loop movements after the binding of the primary substrate, which is hydrophilic. Given that O2 is nonpolar, the increased hydrophobicity at the iron center of the binary complex appears to be essential for rapid O2 binding and activation, explaining the reason for the 3-HAA-induced loop movements. Because substrate binding-induced open-to-closed conformational changes are common, the results reported here may help further our understanding of how oxygen is enriched in nonheme iron-dependent dioxygenases.
Keywords: Extradiol dioxygenase; Nonheme iron enzyme; Oxygen activation; Tryptophan-kynurenine pathway; conformational change; crystal structure; dioxygenase; enzyme structure; hydrophobicity; iron; oxygen binding.
© 2018 Yang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Li X., Guo M., Fan J., Tang W., Wang D., Ge H., Rong H., Teng M., Niu L., Liu Q., and Hao Q. (2006) Crystal structure of 3-hydroxyanthranilic acid 3,4-dioxygenase from Saccharomyces cerevisiae: a special subgroup of the type III extradiol dioxygenases. Protein Sci. 15, 761–773 10.1110/ps.051967906 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

