Cytochrome bd Oxidase Has an Important Role in Sustaining Growth and Development of Streptomyces coelicolor A3(2) under Oxygen-Limiting Conditions
- PMID: 29784883
- PMCID: PMC6060352
- DOI: 10.1128/JB.00239-18
Cytochrome bd Oxidase Has an Important Role in Sustaining Growth and Development of Streptomyces coelicolor A3(2) under Oxygen-Limiting Conditions
Abstract
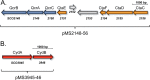
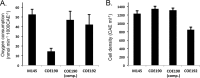
Streptomyces coelicolor A3(2) is a filamentously growing, spore-forming, obligately aerobic actinobacterium that uses both a copper aa3 -type cytochrome c oxidase and a cytochrome bd oxidase to respire oxygen. Using defined knockout mutants, we demonstrated that either of these terminal oxidases was capable of allowing the bacterium to grow and complete its developmental cycle. The genes encoding the bcc complex and the aa3 oxidase are clustered at a single locus. Using Western blot analyses, we showed that the bcc-aa3 oxidase branch is more prevalent in spores than the bd oxidase. The level of the catalytic subunit, CydA, of the bd oxidase was low in spore extracts derived from the wild type, but it was upregulated in a mutant lacking the bcc-aa3 supercomplex. This indicates that cytochrome bd oxidase can compensate for the lack of the other respiratory branch. Components of both oxidases were abundant in growing mycelium. Growth studies in liquid medium revealed that a mutant lacking the bcc-aa3 oxidase branch grew approximately half as fast as the wild type, while the oxygen reduction rate of the mutant remained close to that of the wild type, indicating that the bd oxidase was mainly functioning in controlling electron flux. Developmental defects were observed for a mutant lacking the cytochrome bd oxidase during growth on buffered rich medium plates with glucose as the energy substrate. Evidence based on using the redox-cycling dye methylene blue suggested that cytochrome bd oxidase is essential for the bacterium to grow and complete its developmental cycle under oxygen limitation.IMPORTANCE Respiring with oxygen is an efficient means of conserving energy in biological systems. The spore-forming, filamentous actinobacterium Streptomyces coelicolor grows only aerobically, synthesizing two enzyme complexes for O2 reduction, the cytochrome bcc-aa3 cytochrome oxidase supercomplex and the cytochrome bd oxidase. We show in this study that the bacterium can survive with either of these respiratory pathways to oxygen. Immunological studies indicate that the bcc-aa3 oxidase is the main oxidase present in spores, but the bd oxidase compensates if the bcc-aa3 oxidase is inactivated. Both oxidases are active in mycelia. Growth conditions were identified, revealing that cytochrome bd oxidase is essential for aerial hypha formation and sporulation, and this was linked to an important role of the enzyme under oxygen-limiting conditions.
Keywords: actinobacteria; cytochrome bd oxidase; cytochrome oxidases; menaquinol; mycelium; respiration; spores.
Copyright © 2018 American Society for Microbiology.
Figures




References
-
- Kim MS, Jang J, Ab Rahman NB, Pethe K, Berry EA, Huang LS. 2015. Isolation and characterization of a hybrid respiratory supercomplex consisting of Mycobacterium tuberculosis cytochrome bcc and Mycobacterium smegmatis cytochrome aa3. J Biol Chem 290:14350–14360. doi: 10.1074/jbc.M114.624312. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

