SINHCAF/FAM60A and SIN3A specifically repress HIF-2α expression
- PMID: 29784889
- PMCID: PMC6024822
- DOI: 10.1042/BCJ20170945
SINHCAF/FAM60A and SIN3A specifically repress HIF-2α expression
Abstract
The SIN3A-HDAC (histone deacetylase) complex is a master transcriptional repressor, required for development but often deregulated in disease. Here, we report that the recently identified new component of this complex, SINHCAF (SIN3A and HDAC-associated factor)/FAM60A (family of homology 60A), links the SIN3A-HDAC co-repressor complex function to the hypoxia response. We show that SINHCAF specifically represses HIF-2α mRNA and protein expression, via its interaction with the transcription factor SP1 (specificity protein 1) and recruitment of HDAC1 to the HIF-2α promoter. SINHCAF control over HIF-2α results in functional cellular changes in in vitro angiogenesis and viability. Our analysis reveals an unexpected link between SINHCAF and the regulation of the hypoxia response.
Keywords: HIF-2histone deacetylases; SIN3A; SP1; hypoxia; hypoxia-inducible factors; transcription.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures
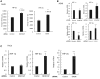

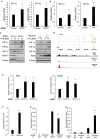
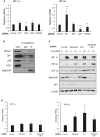
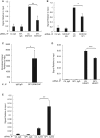
References
-
- Muñoz I.M., MacArtney T., Sanchez-Pulido L., Ponting C.P., Rocha S. and Rouse J. (2012) Family with sequence similarity 60A (FAM60A) protein is a cell cycle-fluctuating regulator of the SIN3-HDAC1 histone deacetylase complex. J. Biol. Chem. 287, 32346–32353 10.1074/jbc.M112.382499 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

