A predictive model for risk of early grade ≥ 3 infection in patients with multiple myeloma not eligible for transplant: analysis of the FIRST trial
- PMID: 29784907
- PMCID: PMC5990520
- DOI: 10.1038/s41375-018-0133-x
A predictive model for risk of early grade ≥ 3 infection in patients with multiple myeloma not eligible for transplant: analysis of the FIRST trial
Abstract
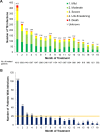
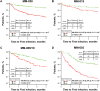
Infections are a major cause of death in patients with multiple myeloma. A post hoc analysis of the phase 3 FIRST trial was conducted to characterize treatment-emergent (TE) infections and study risk factors for TE grade ≥ 3 infection. The number of TE infections/month was highest during the first 4 months of treatment (defined as early infection). Of 1613 treated patients, 340 (21.1%) experienced TE grade ≥ 3 infections in the first 18 months and 56.2% of these patients experienced their first grade ≥ 3 infection in the first 4 months. Risk of early infection was similar regardless of treatment. Based on the analyses of data in 1378 patients through multivariate logistic regression, a predictive model of first TE grade ≥ 3 infection in the first 4 months retained Eastern Cooperative Oncology Group performance status and serum β2-microglobulin, lactate dehydrogenase, and hemoglobin levels to define high- and low-risk groups showing significantly different rates of infection (24.0% vs. 7.0%, respectively; P < 0.0001). The predictive model was validated with data from three clinical trials. This predictive model of early TE grade ≥ 3 infection may be applied in the clinical setting to guide infection monitoring and strategies for infection prevention.
Conflict of interest statement
Charles Dumontet has received honoraria from Sanofi and Janssen, has received fees for a consulting/advisory role from Merck, and has received research funding from Roche. Cyrille Hulin has received honoraria from Celgene, Amgen, Bristol-Myers Squibb, Novartis, Janssen-Cilag, and Takeda. Meletios A. Dimopoulos has received honoraria from Amgen, Celgene, Janssen, and Takeda and has received fees for a consulting/advisory role from Amgen, Celgene, Janssen, and Takeda. Angela Dispenzieri has received research funding from Alnylam, Celgene, Pfizer, Prothena, and Takeda. Heinz Ludwig has received speakers’ bureau fees from Celgene, Janssen, Takeda, and Amgen and has received research funding from Takeda and Amgen. Michele Cavo has received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda. Juan José Lahuerta has received fees for a consulting/advisory role from Celgene, Janssen, and Takeda. Olivier Allangba has received fees for a consulting/advisory role from Novartis and has received travel, accommodations, and expenses from Takeda, Pfizer, Celgene, Amgen, and Roche. Eileen Boyle has received fees for a consulting/advisory role from Celgene. Aurore Perrot has received honoraria and fees for a consulting/advisory role from Celgene, Janssen, Takeda, and Bristol-Myers Squibb. Philippe Moreau has received honoraria from Celgene, Takeda, Novartis, Amgen, and Janssen-Cilag and has received fees for a consulting/advisory role from Celgene, Takeda, Novartis, Amgen, and Janssen. Murielle Roussel has received research funding from Amgen, Celgene, and Janssen. Mohamad Mohty has received honoraria from Celgene, Janssen, Bristol-Myers Squibb, Takeda, Novartis, and Amgen; fees for consulting/advisory role from Celgene, Janssen, Bristol-Myers Squibb, Takeda, Novartis, and Amgen; speakers’ bureau fees from Janssen and Sanofi; research funding from Sanofi; and travel, accommodations, expenses from Sanofi, JAZZ, Novartis, Janssen, and Amgen. Alexandre Civet has received fees for a consulting/advisory role from Celgene. Bruno Costa is an employee of and owns stock in Celgene. Antoine Tinel is an employee of and owns stock in Celgene. Yann Gaston-Mathé is an employee of IntegraGen, has received fees for a consulting/advisory role from Celgene, and has received travel, accommodations, expenses from Celgene. Thierry Facon has received fees for a consulting/advisory role from Amgen, Celgene, Janssen, Karyopharm, PharmaMar, and Takeda and has received speakers’ bureau fees from Amgen, Celgene, Janssen, and Takeda. Andrew Belch, Philippe Rodon, Jan Van Droogenbroeck, Lugui Qiu, Ann Van de Velde, Jae Hoon Lee, Salomon Manier, Michel Attal, and Jean Yves Mary declare that they have no conflict of interest.
Figures
References
-
- Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–26. doi: 10.1200/JCO.2005.03.2086. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

