A causal mechanism for childhood acute lymphoblastic leukaemia
- PMID: 29784935
- PMCID: PMC6986894
- DOI: 10.1038/s41568-018-0015-6
A causal mechanism for childhood acute lymphoblastic leukaemia
Erratum in
-
Author Correction: A causal mechanism for childhood acute lymphoblastic leukaemia.Nat Rev Cancer. 2018 Aug;18(8):526. doi: 10.1038/s41568-018-0029-0. Nat Rev Cancer. 2018. PMID: 29849071
Abstract
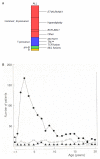
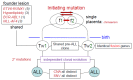
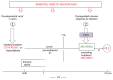
In this Review, I present evidence supporting a multifactorial causation of childhood acute lymphoblastic leukaemia (ALL), a major subtype of paediatric cancer. ALL evolves in two discrete steps. First, in utero initiation by fusion gene formation or hyperdiploidy generates a covert, pre-leukaemic clone. Second, in a small fraction of these cases, the postnatal acquisition of secondary genetic changes (primarily V(D)J recombination-activating protein (RAG) and activation-induced cytidine deaminase (AID)-driven copy number alterations in the case of ETS translocation variant 6 (ETV6)-runt-related transcription factor 1 (RUNX1)+ ALL) drives conversion to overt leukaemia. Epidemiological and modelling studies endorse a dual role for common infections. Microbial exposures earlier in life are protective but, in their absence, later infections trigger the critical secondary mutations. Risk is further modified by inherited genetics, chance and, probably, diet. Childhood ALL can be viewed as a paradoxical consequence of progress in modern societies, where behavioural changes have restrained early microbial exposure. This engenders an evolutionary mismatch between historical adaptations of the immune system and contemporary lifestyles. Childhood ALL may be a preventable cancer.
Conflict of interest statement
The author declares no competing interests.
Figures
References
-
- Parkin DM, et al. Vol. 87 IARC Scientific Publications; Lyon: 1988.
-
- Pinkel D. In: White Blood. Personal journeys with childhood leukaemia. Greaves M, editor. World Scientific; 2008. pp. 13–46.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

