Targeting skeletal endothelium to ameliorate bone loss
- PMID: 29785024
- PMCID: PMC5992080
- DOI: 10.1038/s41591-018-0020-z
Targeting skeletal endothelium to ameliorate bone loss
Abstract
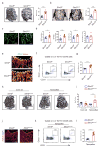
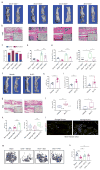
Recent studies have identified a specialized subset of CD31hiendomucinhi (CD31hiEMCNhi) vascular endothelium that positively regulates bone formation. However, it remains unclear how CD31hiEMCNhi endothelium levels are coupled to anabolic bone formation. Mice with an osteoblast-specific deletion of Shn3, which have markedly elevated bone formation, demonstrated an increase in CD31hiEMCNhi endothelium. Transcriptomic analysis identified SLIT3 as an osteoblast-derived, SHN3-regulated proangiogenic factor. Genetic deletion of Slit3 reduced skeletal CD31hiEMCNhi endothelium, resulted in low bone mass because of impaired bone formation and partially reversed the high bone mass phenotype of Shn3-/- mice. This coupling between osteoblasts and CD31hiEMCNhi endothelium is essential for bone healing, as shown by defective fracture repair in SLIT3-mutant mice and enhanced fracture repair in SHN3-mutant mice. Finally, administration of recombinant SLIT3 both enhanced bone fracture healing and counteracted bone loss in a mouse model of postmenopausal osteoporosis. Thus, drugs that target the SLIT3 pathway may represent a new approach for vascular-targeted osteoanabolic therapy to treat bone loss.
Conflict of interest statement
LHG is on the board of directors of and holds equity in the GlaxoSmithKline and Waters Corporations. She is also a founder of Quentis Pharmaceuticals.
Figures




Comment in
-
Endothelium-osteoblast crosstalk.Nat Rev Rheumatol. 2018 Jul;14(7):386. doi: 10.1038/s41584-018-0034-4. Nat Rev Rheumatol. 2018. PMID: 29875380 No abstract available.
-
New horizons for osteoanabolic treatment?Nat Rev Endocrinol. 2018 Sep;14(9):508-509. doi: 10.1038/s41574-018-0069-2. Nat Rev Endocrinol. 2018. PMID: 30050157 No abstract available.
References
-
- Jones DC, et al. Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science. 2006;312:1223–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

