Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning
- PMID: 29785514
- PMCID: PMC6716520
- DOI: 10.1007/s00259-018-4044-x
Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning
Abstract
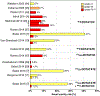
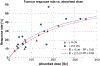
Purpose: Peptide receptor radionuclide therapy (PRRT) with 90Y-labelled and 177Lu-labelled peptides is an effective strategy for the treatment of metastatic/nonresectable neuroendocrine tumours (NETs). Dosimetry provides important information useful for optimizing PRRT with individualized regimens to reduce toxicity and increase tumour responses. However, this strategy is not applied in routine clinical practice, despite the fact that several dosimetric studies have demonstrated significant dose-effect correlations for normal organ toxicity and tumour response that can better guide therapy planning. The present study reviews the key relationships and the radiobiological models available in the literature with the aim of providing evidence that optimization of PRRT is feasible through the implementation of dosimetry.
Methods: The MEDLINE database was searched combining specific keywords. Original studies published in the English language reporting dose-effect outcomes in patients treated with PRRT were chosen.
Results: Nine of 126 studies were selected from PubMed, and a further five were added manually, reporting on 590 patients. The studies were analysed and are discussed in terms of weak and strong elements of correlations.
Conclusion: Several studies provided evidence of clinical benefit from the implementation of dosimetry in PRRT, indicating the potential contribution of this approach to reducing severe toxicity and/or reducing undertreatment that commonly occurs. Prospective trials, possibly multicentre, with larger numbers of patients undergoing quantitative dosimetry and with standardized methodologies should be carried out to definitively provide robust predictive paradigms to establish effective tailored PRRT.
Keywords: Absorbed dose correlations; Dose–effect; Dose–toxicity; Dosimetry; Peptide receptor radionuclide therapy (PRRT).
Conflict of interest statement
C.C. was a consultant for Alfasigma and discloses funding from BTG Biocompatibles for work not related to the present paper.
M.P. was a consultant for Bayer Healthcare Pharmaceuticals Inc. as a member of the Targeted Alpha Therapy Working Group Advisory Board.
M.C., M.E.F., A.S., C.G., L.S., P.E.S., R.O., C.M.G. and F.B. declare that they have no conflicts of interest.
Figures



References
-
- Bodei L, Kwekkeboom DJ, Kidd M, Modlin IM, Krenning EP. Radiolabeled somatostatin analogue therapy of gastroenteropancreatic cancer. Semin Nucl Med. 2016;46(3):225–38. - PubMed
-
- Cybulla M, Weiner SM, Otte A. End-stage renal disease after treatment with 90Y-DOTATOC. Eur J Nucl Med. 2001;28(10):1552–4. - PubMed
-
- Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–23. - PubMed
-
- Gupta SK, Singla S, Bal C. Renal and hematological toxicity in patients of neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-DOTATATE. Cancer Biother Radiopharm. 2012;27(9):593–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

