Mechanism of selective recruitment of RNA polymerases II and III to snRNA gene promoters
- PMID: 29785964
- PMCID: PMC6004067
- DOI: 10.1101/gad.314245.118
Mechanism of selective recruitment of RNA polymerases II and III to snRNA gene promoters
Abstract
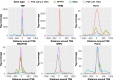
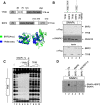
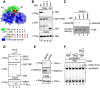
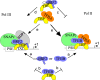
RNA polymerase II (Pol II) small nuclear RNA (snRNA) promoters and type 3 Pol III promoters have highly similar structures; both contain an interchangeable enhancer and "proximal sequence element" (PSE), which recruits the SNAP complex (SNAPc). The main distinguishing feature is the presence, in the type 3 promoters only, of a TATA box, which determines Pol III specificity. To understand the mechanism by which the absence or presence of a TATA box results in specific Pol recruitment, we examined how SNAPc and general transcription factors required for Pol II or Pol III transcription of SNAPc-dependent genes (i.e., TATA-box-binding protein [TBP], TFIIB, and TFIIA for Pol II transcription and TBP and BRF2 for Pol III transcription) assemble to ensure specific Pol recruitment. TFIIB and BRF2 could each, in a mutually exclusive fashion, be recruited to SNAPc. In contrast, TBP-TFIIB and TBP-BRF2 complexes were not recruited unless a TATA box was present, which allowed selective and efficient recruitment of the TBP-BRF2 complex. Thus, TBP both prevented BRF2 recruitment to Pol II promoters and enhanced BRF2 recruitment to Pol III promoters. On Pol II promoters, TBP recruitment was separate from TFIIB recruitment and enhanced by TFIIA. Our results provide a model for specific Pol recruitment at SNAPc-dependent promoters.
Keywords: BRF2; SNAPc; TBP; TFIIA; TFIIB; small nuclear RNA promoters.
© 2018 Dergai et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



Similar articles
-
Role of the inhibitory DNA-binding surface of human TATA-binding protein in recruitment of human TFIIB family members.Mol Cell Biol. 2003 Nov;23(22):8152-60. doi: 10.1128/MCB.23.22.8152-8160.2003. Mol Cell Biol. 2003. PMID: 14585974 Free PMC article.
-
Structure-function analysis of the human TFIIB-related factor II protein reveals an essential role for the C-terminal domain in RNA polymerase III transcription.Mol Cell Biol. 2005 Nov;25(21):9406-18. doi: 10.1128/MCB.25.21.9406-9418.2005. Mol Cell Biol. 2005. PMID: 16227591 Free PMC article.
-
Structural basis of SNAPc-dependent snRNA transcription initiation by RNA polymerase II.Nat Struct Mol Biol. 2022 Dec;29(12):1159-1169. doi: 10.1038/s41594-022-00857-w. Epub 2022 Nov 24. Nat Struct Mol Biol. 2022. PMID: 36424526 Free PMC article.
-
Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.Gene. 2022 Jul 30;833:146581. doi: 10.1016/j.gene.2022.146581. Epub 2022 May 18. Gene. 2022. PMID: 35597524 Review.
-
Regulation of snRNA gene expression by the Drosophila melanogaster small nuclear RNA activating protein complex (DmSNAPc).Crit Rev Biochem Mol Biol. 2011 Feb;46(1):11-26. doi: 10.3109/10409238.2010.518136. Epub 2010 Oct 6. Crit Rev Biochem Mol Biol. 2011. PMID: 20925482 Review.
Cited by
-
Evolution of plant telomerase RNAs: farther to the past, deeper to the roots.Nucleic Acids Res. 2021 Jul 21;49(13):7680-7694. doi: 10.1093/nar/gkab545. Nucleic Acids Res. 2021. PMID: 34181710 Free PMC article.
-
Never a dull enzyme, RNA polymerase II.Transcription. 2023 Nov;14(1-2):49-67. doi: 10.1080/21541264.2023.2208023. Epub 2023 May 2. Transcription. 2023. PMID: 37132022 Free PMC article. Review.
-
Molecular basis of neurodegeneration in a mouse model of Polr3-related disease.Elife. 2024 Nov 5;13:RP95314. doi: 10.7554/eLife.95314. Elife. 2024. PMID: 39499645 Free PMC article.
-
Structural insights into distinct mechanisms of RNA polymerase II and III recruitment to snRNA promoters.Nat Commun. 2025 Jan 2;16(1):141. doi: 10.1038/s41467-024-55553-8. Nat Commun. 2025. PMID: 39747245 Free PMC article.
-
The Integrator complex: an emerging complex structure involved in the regulation of gene expression by targeting RNA polymerase II.Funct Integr Genomics. 2024 Oct 19;24(6):192. doi: 10.1007/s10142-024-01479-9. Funct Integr Genomics. 2024. PMID: 39424688 Review.
References
-
- Abascal-Palacios G, Ramsay EP, Beuron F, Morris E, Vannini A. 2018. Structural basis of RNA polymerase III transcription initiation. Nature 553: 301–306. - PubMed
-
- Emran F, Florens L, Ma B, Swanson SK, Washburn MP, Hernandez N. 2006. A role for Yin Yang-1 (YY1) in the assembly of snRNA transcription complexes. Gene 377: 96–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
