MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration
- PMID: 29786072
- PMCID: PMC6329843
- DOI: 10.1038/s41418-018-0124-5
MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration
Abstract
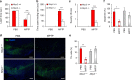
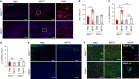
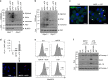
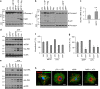
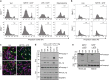
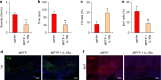
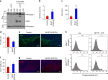
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra (SN) and the reduction of dopamine levels in the striatum. Although details of the molecular mechanisms underlying dopaminergic neuronal death in PD remain unclear, neuroinflammation is also considered a potent mediator in the pathogenesis and progression of PD. In the present study, we present evidences that microglial NLRP3 inflammasome activation is critical for dopaminergic neuronal loss and the subsequent motor deficits in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. Specifically, NLRP3 deficiency significantly reduces motor dysfunctions and dopaminergic neurodegeneration of MPTP-treated mice. Furthermore, NLRP3 deficiency abolishes MPTP-induced microglial recruitment, interleukin-1β production and caspase-1 activation in the SN of mouse brain. In primary microglia and mixed glial cell cultures, MPTP/ATP treatment promotes the robust assembly and activation of the NLRP3 inflammasome via producing mitochondrial reactive oxygen species. Consistently, 1-methyl-4-phenyl-pyridinium (MPP+) induces NLRP3 inflammasome activation in the presence of ATP or nigericin treatment in mouse bone-marrow-derived macrophages. These findings reveal a novel priming role of neurotoxin MPTP or MPP+ for NLRP3 activation. Subsequently, NLRP3 inflammasome-active microglia induces profound neuronal death in a microglia-neuron co-culture model. Furthermore, Cx3Cr1CreER-based microglia-specific expression of an active NLRP3 mutant greatly exacerbates motor deficits and dopaminergic neuronal loss of MPTP-treated mice. Taken together, our results indicate that microglial NLRP3 inflammasome activation plays a pivotal role in the MPTP-induced neurodegeneration in PD.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

