c-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs
- PMID: 29786076
- PMCID: PMC6370811
- DOI: 10.1038/s41418-018-0129-0
c-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs
Abstract
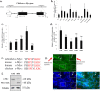
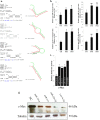
The transcription factor c-Myc is an important regulator of cellular proliferation, differentiation and embryogenesis. While c-Myc can inhibit myoblast differentiation, the underlying mechanisms remain poorly understood. Here, we found that c-Myc does not only inhibits myoblast differentiation but also promotes myoblast proliferation and muscle fibre hypertrophy. By performing chromatin immunoprecipitation and high-throughput sequencing (ChIP-seq), we identified the genome-wide binding profile of c-Myc in skeletal muscle cells. c-Myc achieves its regulatory effects on myoblast proliferation and differentiation by targeting the cell cycle pathway. Additionally, c-Myc can regulate cell cycle genes by controlling miRNA expression of which dozens of miRNAs can also be regulated directly by c-Myc. Among these c-Myc-associated miRNAs (CAMs), the roles played by c-Myc-induced miRNAs in skeletal muscle cells are similar to those played by c-Myc, whereas c-Myc-repressed miRNAs play roles that are opposite to those played by c-Myc. The cell cycle, ERK-MAPK and Akt-mediated pathways are potential target pathways of the CAMs during myoblast differentiation. Interestingly, we identified four CAMs that can directly bind to the c-Myc 3' UTR and inhibit c-Myc expression, suggesting that a negative feedback loop exists between c-Myc and its target miRNAs during myoblast differentiation. c-Myc also potentially regulates many long intergenic noncoding RNAs (lincRNAs). Linc-2949 and linc-1369 are directly regulated by c-Myc, and both lincRNAs are involved in the regulation of myoblast proliferation and differentiation by competing for the binding of muscle differentiation-related miRNAs. Our findings do not only provide a genome-wide overview of the role the c-Myc plays in skeletal muscle cells but also uncover the mechanism of how c-Myc and its target genes regulate myoblast proliferation and differentiation, and muscle fibre hypertrophy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
MicroRNA-664-5p promotes myoblast proliferation and inhibits myoblast differentiation by targeting serum response factor and Wnt1.J Biol Chem. 2018 Dec 14;293(50):19177-19190. doi: 10.1074/jbc.RA118.003198. Epub 2018 Oct 15. J Biol Chem. 2018. PMID: 30323063 Free PMC article.
-
Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis.J Cell Sci. 2013 Jun 15;126(Pt 12):2678-91. doi: 10.1242/jcs.119966. Epub 2013 Apr 19. J Cell Sci. 2013. PMID: 23606743 Free PMC article.
-
Linc-smad7 promotes myoblast differentiation and muscle regeneration via sponging miR-125b.Epigenetics. 2018;13(6):591-604. doi: 10.1080/15592294.2018.1481705. Epub 2018 Aug 6. Epigenetics. 2018. PMID: 29912619 Free PMC article.
-
CDR1as/miRNAs-related regulatory mechanisms in muscle development and diseases.Gene. 2020 Mar 10;730:144315. doi: 10.1016/j.gene.2019.144315. Epub 2020 Jan 2. Gene. 2020. PMID: 31904497 Review.
-
MicroRNAs involved in skeletal muscle differentiation.J Genet Genomics. 2013 Mar 20;40(3):107-16. doi: 10.1016/j.jgg.2013.02.002. Epub 2013 Feb 20. J Genet Genomics. 2013. PMID: 23522383 Review.
Cited by
-
Farnesoid-X receptor as a therapeutic target for inflammatory bowel disease and colorectal cancer.Front Pharmacol. 2022 Oct 6;13:1016836. doi: 10.3389/fphar.2022.1016836. eCollection 2022. Front Pharmacol. 2022. PMID: 36278234 Free PMC article. Review.
-
Dissecting role of founder mutation p.V727M in GNE in Indian HIBM cohort.Open Med (Wars). 2021 Nov 15;16(1):1733-1744. doi: 10.1515/med-2021-0391. eCollection 2021. Open Med (Wars). 2021. PMID: 34825065 Free PMC article.
-
Inferring gene regulatory networks from single-cell transcriptomics based on graph embedding.Bioinformatics. 2024 May 2;40(5):btae291. doi: 10.1093/bioinformatics/btae291. Bioinformatics. 2024. PMID: 38810116 Free PMC article.
-
Signaling pathways and regulatory networks in quail skeletal muscle development: insights from whole transcriptome sequencing.Poult Sci. 2024 May;103(5):103603. doi: 10.1016/j.psj.2024.103603. Epub 2024 Mar 1. Poult Sci. 2024. PMID: 38457990 Free PMC article.
-
Effect of miR-184 on Proliferation and Apoptosis of Pancreatic Ductal Adenocarcinoma and Its Mechanism.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820943237. doi: 10.1177/1533033820943237. Technol Cancer Res Treat. 2020. PMID: 32914707 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

