Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex
- PMID: 29786081
- PMCID: PMC6390950
- DOI: 10.1038/s41593-018-0143-z
Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex
Abstract
How learning enhances neural representations for behaviorally relevant stimuli via activity changes of cortical cell types remains unclear. We simultaneously imaged responses of pyramidal cells (PYR) along with parvalbumin (PV), somatostatin (SOM), and vasoactive intestinal peptide (VIP) inhibitory interneurons in primary visual cortex while mice learned to discriminate visual patterns. Learning increased selectivity for task-relevant stimuli of PYR, PV and SOM subsets but not VIP cells. Strikingly, PV neurons became as selective as PYR cells, and their functional interactions reorganized, leading to the emergence of stimulus-selective PYR-PV ensembles. Conversely, SOM activity became strongly decorrelated from the network, and PYR-SOM coupling before learning predicted selectivity increases in individual PYR cells. Thus, learning differentially shapes the activity and interactions of multiple cell classes: while SOM inhibition may gate selectivity changes, PV interneurons become recruited into stimulus-specific ensembles and provide more selective inhibition as the network becomes better at discriminating behaviorally relevant stimuli.
Figures
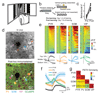



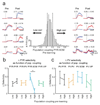
References
-
- Chen JL, Margolis DJ, Stankov A, Sumanovski LT, Schneider BL, Helmchen F. Pathway-specific reorganization of projection neurons in somatosensory cortex during learning. Nat Neurosci. 2015a;18:1101–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

