Temperature Effects on Force and Actin⁻Myosin Interaction in Muscle: A Look Back on Some Experimental Findings
- PMID: 29786656
- PMCID: PMC5983754
- DOI: 10.3390/ijms19051538
Temperature Effects on Force and Actin⁻Myosin Interaction in Muscle: A Look Back on Some Experimental Findings
Abstract
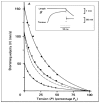
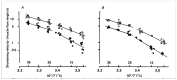
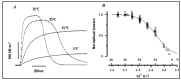
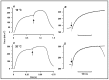
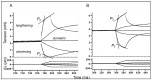
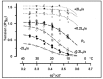
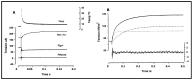
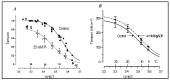
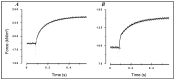
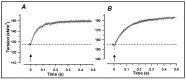
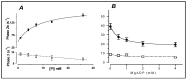
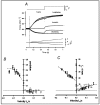
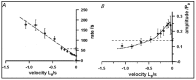
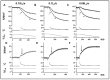
Observations made in temperature studies on mammalian muscle during force development, shortening, and lengthening, are re-examined. The isometric force in active muscle goes up substantially on warming from less than 10 °C to temperatures closer to physiological (>30 °C), and the sigmoidal temperature dependence of this force has a half-maximum at ~10 °C. During steady shortening, when force is decreased to a steady level, the sigmoidal curve is more pronounced and shifted to higher temperatures, whereas, in lengthening muscle, the curve is shifted to lower temperatures, and there is a less marked increase with temperature. Even with a small rapid temperature-jump (T-jump), force in active muscle rises in a definitive way. The rate of tension rise is slower with adenosine diphosphate (ADP) and faster with increased phosphate. Analysis showed that a T-jump enhances an early, pre-phosphate release step in the acto-myosin (crossbridge) ATPase cycle, thus inducing a force-rise. The sigmoidal dependence of steady force on temperature is due to this endothermic nature of crossbridge force generation. During shortening, the force-generating step and the ATPase cycle are accelerated, whereas during lengthening, they are inhibited. The endothermic force generation is seen in different muscle types (fast, slow, and cardiac). The underlying mechanism may involve a structural change in attached myosin heads and/or their attachments on heat absorption.
Keywords: actin–myosin; crossbridge cycle; crossbridge force; endothermic force; muscle force; muscle shortening; temperature-sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Crossbridge mechanism(s) examined by temperature perturbation studies on muscle.Adv Exp Med Biol. 2010;682:247-66. doi: 10.1007/978-1-4419-6366-6_14. Adv Exp Med Biol. 2010. PMID: 20824530 Review.
-
Force generation examined by laser temperature-jumps in shortening and lengthening mammalian (rabbit psoas) muscle fibres.J Physiol. 2007 Nov 15;585(Pt 1):263-77. doi: 10.1113/jphysiol.2007.142208. Epub 2007 Oct 4. J Physiol. 2007. PMID: 17916609 Free PMC article.
-
The endothermic ATP hydrolysis and crossbridge attachment steps drive the increase of force with temperature in isometric and shortening muscle.J Physiol. 2015 Apr 15;593(8):1997-2016. doi: 10.1113/jphysiol.2014.284992. Epub 2015 Feb 11. J Physiol. 2015. PMID: 25564737 Free PMC article.
-
Force and power generating mechanism(s) in active muscle as revealed from temperature perturbation studies.J Physiol. 2010 Oct 1;588(Pt 19):3657-70. doi: 10.1113/jphysiol.2010.194001. J Physiol. 2010. PMID: 20660565 Free PMC article. Review.
-
Endothermic force generation, temperature-jump experiments and effects of increased [MgADP] in rabbit psoas muscle fibres.J Physiol. 2005 Sep 1;567(Pt 2):471-92. doi: 10.1113/jphysiol.2005.090084. Epub 2005 Jun 23. J Physiol. 2005. PMID: 15975981 Free PMC article.
Cited by
-
Opto-thermal technologies for microscopic analysis of cellular temperature-sensing systems.Biophys Rev. 2021 Nov 3;14(1):41-54. doi: 10.1007/s12551-021-00854-1. eCollection 2022 Feb. Biophys Rev. 2021. PMID: 35340595 Free PMC article. Review.
-
Effects of Hydrostatic-Pressure on Muscle Contraction: A Look Back on Some Experimental Findings.Int J Mol Sci. 2023 Mar 6;24(5):5031. doi: 10.3390/ijms24055031. Int J Mol Sci. 2023. PMID: 36902460 Free PMC article. Review.
-
Non-cross Bridge Viscoelastic Elements Contribute to Muscle Force and Work During Stretch-Shortening Cycles: Evidence From Whole Muscles and Permeabilized Fibers.Front Physiol. 2021 Mar 29;12:648019. doi: 10.3389/fphys.2021.648019. eCollection 2021. Front Physiol. 2021. PMID: 33854441 Free PMC article.
-
Influence and Mechanisms of Action of Environmental Stimuli on Work Near and Above the Severe Domain Boundary (Critical Power).Sports Med Open. 2022 Mar 28;8(1):42. doi: 10.1186/s40798-022-00430-1. Sports Med Open. 2022. PMID: 35347469 Free PMC article. Review.
-
The likely effects of thermal climate change on vertebrate skeletal muscle mechanics with possible consequences for animal movement and behaviour.Conserv Physiol. 2019 Oct 31;7(1):coz066. doi: 10.1093/conphys/coz066. eCollection 2019. Conserv Physiol. 2019. PMID: 31687144 Free PMC article. Review.
References
-
- Huxley A.F. Muscle structure and theories of contraction. Prog. Biophys. 1957;7:285–318. - PubMed
-
- Sugi H. Evidence for the essential role of myosin subfragment-2 in muscle contraction: Functional communication between myosin head and subfragment-2. J. Mater. Sci. Eng. 2017;6:386. doi: 10.4172/2169-0022.1000386. - DOI
-
- Hill A.V. The influence of temperature on the tension developed in an isometric twitch. Proc. Soc. Lond. B. 1951;138:349–354. doi: 10.1098/rspb.1951.0028. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

