A Framework for Image-Based Modeling of Acute Myocardial Ischemia Using Intramurally Recorded Extracellular Potentials
- PMID: 29786776
- PMCID: PMC6202115
- DOI: 10.1007/s10439-018-2048-0
A Framework for Image-Based Modeling of Acute Myocardial Ischemia Using Intramurally Recorded Extracellular Potentials
Abstract
The biophysical basis for electrocardiographic evaluation of myocardial ischemia stems from the notion that ischemic tissues develop, with relative uniformity, along the endocardial aspects of the heart. These injured regions of subendocardial tissue give rise to intramural currents that lead to ST segment deflections within electrocardiogram (ECG) recordings. The concept of subendocardial ischemic regions is often used in clinical practice, providing a simple and intuitive description of ischemic injury; however, such a model grossly oversimplifies the presentation of ischemic disease-inadvertently leading to errors in ECG-based diagnoses. Furthermore, recent experimental studies have brought into question the subendocardial ischemia paradigm suggesting instead a more distributed pattern of tissue injury. These findings come from experiments and so have both the impact and the limitations of measurements from living organisms. Computer models have often been employed to overcome the constraints of experimental approaches and have a robust history in cardiac simulation. To this end, we have developed a computational simulation framework aimed at elucidating the effects of ischemia on measurable cardiac potentials. To validate our framework, we simulated, visualized, and analyzed 226 experimentally derived acute myocardial ischemic events. Simulation outcomes agreed both qualitatively (feature comparison) and quantitatively (correlation, average error, and significance) with experimentally obtained epicardial measurements, particularly under conditions of elevated ischemic stress. Our simulation framework introduces a novel approach to incorporating subject-specific, geometric models and experimental results that are highly resolved in space and time into computational models. We propose this framework as a means to advance the understanding of the underlying mechanisms of ischemic disease while simultaneously putting in place the computational infrastructure necessary to study and improve ischemia models aimed at reducing diagnostic errors in the clinic.
Keywords: Cardiac simulation; Computer model; Electrocardiographic forward problem; Extracellular potentials; Ischemia; ST deviation.
Figures





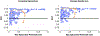

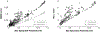
References
-
- Akkerhuis K and Simoons M. Exercise electrocardiography and exercise testing In: Comprehensive Electrocardiology, Vol. 1, chapter 36, edited by Macfarlane P, van Oosterom A, Pahlm O, Kligfield P, Janse M, and Camm J, 2nd ed. London, England: Springer, 2011, pp. 1677–1719.
-
- Arisi G, Macchi E, Corradi C, Lux R, and Taccardi B. Epicardial excitation during ventricular pacing: Relative independence of breakthrough sites from excitation sequence in canine right ventricle. Circ. Res 71:840–849, 1992. - PubMed
-
- Callahan M, Cole M, Shepherd J, Stinstra J, and Johnson C. BioMesh3D: A meshing pipeline for biomedical models SCI Institute Technical Report UUSCI-2007-009, University of Utah, 2007.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

