Oral contraceptives for pain associated with endometriosis
- PMID: 29786828
- PMCID: PMC6494634
- DOI: 10.1002/14651858.CD001019.pub3
Oral contraceptives for pain associated with endometriosis
Abstract
Background: Endometriosis is a common gynaecological condition which affects many women of reproductive age worldwide and is a major cause of pain and infertility. The combined oral contraceptive pill (COCP) is widely used to treat pain occurring as a result of endometriosis, although the evidence for its efficacy is limited.
Objectives: To determine the effectiveness, safety and cost-effectiveness of oral contraceptive preparations in the treatment of painful symptoms ascribed to the diagnosis of laparoscopically proven endometriosis.
Search methods: We searched the following from inception to 19 October 2017: the Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, the Cochrane CENTRAL Register of Studies Online (CRSO), MEDLINE, Embase, PsycINFO, CINAHL (Cumulative Index to Nursing and Allied Health Literature), and the trial registers ClinicalTrials.gov and the World Health Organization Clinical Trials Registry Platform (WHO ICTRP). We also handsearched reference lists of relevant trials and systematic reviews retrieved by the search.
Selection criteria: We included randomised controlled trials (RCT) of the use of COCPs in the treatment of women of reproductive age with symptoms ascribed to the diagnosis of endometriosis that had been made visually at a surgical procedure.
Data collection and analysis: Two review authors independently assessed study quality and extracted data. One review author was an expert in the content matter. We contacted study authors for additional information. The primary outcome was self-reported pain (dysmenorrhoea) at the end of treatment.
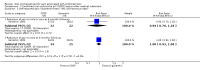
Main results: Five trials (612 women) met the inclusion criteria. Only three trials (404 women) provided data that were suitable for analysis.Combined oral contraceptive pill versus placeboTwo trials compared COCP with a placebo. These studies were at high risk of bias. For GRADE outcomes (self-reported pain (dysmenorrhoea) at the end of treatment), the quality of the evidence very low. Evidence was downgraded for imprecision as it was based on a single, small trial and for the visual analogue scale data there were wide confidence intervals (CIs). There appeared to have been substantial involvement of the pharmaceutical company funding the trials.Treatment with the COCP was associated with an improvement in self-reported pain at the end of treatment as evidenced by a lower score on the Dysmenorrhoea verbal rating scale (scale 0 to 3) compared with placebo (mean difference (MD) -1.30 points, 95% CI -1.84 to -0.76; 1 RCT, 96 women; very low quality evidence), a lower score on the Dysmenorrhoea visual analogue scale (no details of scale) compared with placebo (MD -23.68 points, 95% CI -28.75 to -18.62, 2 RCTs, 327 women; very low quality evidence) and a reduction in menstrual pain from baseline to the end of treatment (MD 2.10 points, 95% CI 1.38 to 2.82; 1 RCT, 169 women; very low quality evidence).Combined oral contraceptive pill versus medical therapiesOne underpowered trial compared the COCP with another medical treatment (goserelin). The study was at high risk of bias; the trial was unblinded and there was insufficient detail to judge allocation concealment and randomisation. For GRADE outcomes (self-reported pain (dysmenorrhoea) at the end of treatment), the quality of the evidence ranged from low to very low.At the end of treatment, the women in the goserelin group were amenorrhoeic and therefore no comparisons could be made between the groups for the primary outcome. At six months' follow-up, there was no clear evidence of a difference between women treated with the COCP and women treated with goserelin for measures of dysmenorrhoea on a visual analogue scale (scale 1 to 10) (MD -0.10, 95% CI -1.28 to 1.08; 1 RCT, 50 women; very low quality evidence) or a verbal rating scale (scale 0 to 3) (MD -0.10, 95% CI -0.99 to 0.79; 1 RCT, 50 women; very low quality evidence). At six months' follow-up, there was no clear evidence of a difference between the COCP and goserelin groups for reporting complete absence of pain as measured by the visual analogue scale (risk ratio (RR) 0.36, 95% CI 0.02 to 8.43; 1 RCT, 50 women; very low quality evidence) or the verbal rating scale (RR 1.00, 95% CI 0.93 to 1.08; 1 RCT, 49 women; low quality evidence).
Authors' conclusions: Based on the limited evidence from two trials at high risk of bias and limited data for the prespecified outcomes for this review, there is insufficient evidence to make a judgement on the effectiveness of the COCP compared with placebo and the findings cannot be generalised.Based on the limited evidence from one small trial that was at high risk of bias, there is insufficient evidence to make a judgement on the effectiveness of the COCP compared with other medical treatments. Only one comparison was possible, with the medical intervention being goserelin, and the findings cannot be generalised.Further research is needed to fully evaluate the role of COCPs in managing pain-related symptoms associated with endometriosis. There are other formulations of the combined hormonal contraception such as the transdermal patch, vaginal ring or combined injectable contraceptives which this review did not cover but should be considered in future updates.
Conflict of interest statement
JB: none known.
TC: none known.
SD: none known.
AP: none known.
Figures






















Update of
-
Oral contraceptives for pain associated with endometriosis.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019. doi: 10.1002/14651858.CD001019.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2018 May 22;5:CD001019. doi: 10.1002/14651858.CD001019.pub3. PMID: 17636650 Updated.
References
References to studies included in this review
Ali 2013 {published data only}
-
- Ali AF, Farid LA, Fouad M, Omar ED. Continuous oral contraception and leuprolide in the treatment of endometriosis associated pelvic pain. Journal of Endometriosis 2013;5 (Suppl 1).
-
- Ali AFM, Farid LA, Fouad M, Daly O. Continuous oral contraception and leuprolide in the treatment of endometriosis‐associated pelvic pain. European Journal of Contraception and Reproductive Health Care 2013;18 (Suppl 1):S257.
Guzick 2011 {published data only}
Harada 2008 {published data only}
-
- Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low‐dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo‐controlled, double‐blind, randomized trial. Fertility and Sterility 2008;90(5):1583‐88. - PubMed
Harada 2017 {published data only}
-
- Harada T, Kosaka S, Ellisen J, Yasuda M, Ito M, Momoeda M. Ethinylestradiol 20 μg/drospireone 3 mg in a flexible extended regiment for the management of endometriosis‐associated pelvic pain: a randomized controlled trial. Fertility and Sterility 2017;108(5):798‐805. [DOI: 10.1016/j.fertstert.2017.07.1165] - DOI - PubMed
Vercellini 1993 {published data only}
-
- Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotrophin‐releasing hormone agonist versus a low‐dose oral contraceptive for pelvic pain associated with endometriosis. Fertility and Sterility 1993;60(1):75‐9. - PubMed
References to studies excluded from this review
Caruso 2011 {published data only}
-
- Caruso S, Iraci Sareri M, Agnello C, Romano M, Lo Presti L, Malandrino C, et al. Conventional vs. extended‐cycle oral contraceptives on the quality of sexual life: comparison between two regimens containing 3 mg drospirenone and 20 micro g ethinyl estradiol. Journal of Sexual Medicine 2011;8(5):1478‐85. - PubMed
Caruso 2016 {published data only}
-
- Caruso S, Iraci M, Cianci S, Fava V, Casella E, Cianci A. Comparative, open‐label prospective study on the quality of life and sexual function of women affected by endometriosis‐associated pelvic pain on 2 mg dienogest/30 micro g ethinyl estradiol continuous or 21/7 regimen oral contraceptive. Journal of Endocrinological Investigation 2016;39(8):923‐31. - PubMed
Cheewadhanaraks 2012 {published data only}
-
- Cheewadhanaraks S, Choksuchat C, Dhanaworavibul K, Liabsuetrakul T. Postoperative depot medroxyprogesterone acetate versus continuous oral contraceptive pills in the treatment of endometriosis‐associated pain: a randomized comparative trial. Gynecologic and Obstetric Investigation 2012;74(2):151‐6. - PubMed
Cucinella 2013 {published data only}
-
- Cucinella G, Granese R, Calagna G, Svelato A, Saitta S, Tonni G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference?. Archives of Gynecology & Obstetrics 2013;288(4):821‐7. - PubMed
Fedele 1989 {published data only}
-
- Fedele L, Arcaini L, Bianchi S, Baglioni A, Vercillini P. Comparison of cyproterone acetate and danazol in the treatment of pelvic pain associated with endometriosis. Obstetrics and Gynecology 1989;73(6):1000‐4. - PubMed
Fedele 2008 {published data only}
-
- Fedele L, Bianchi S, Montefusco S, Frontino G, Carmignani L. A gonadotropin‐releasing hormone agonist versus a continuous oral contraceptive pill in the treatment of bladder endometriosis. Fertility and Sterility 2008;90(1):183‐4. - PubMed
Granese 2015 {published data only}
-
- Granese R, Perino A, Calagna G, Saitta S, Franciscis P, Colacurci N, et al. Gonadotrophin‐releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi‐center randomized trial. Acta Obstetricia et Gynecologica Scandinavica 2015;94(6):637‐45. - PubMed
Kitawaki 2012 {published and unpublished data}
-
- Kitawaki J, Kusuki I, Suganuma I, Sasaki A, Itoh F, Matsuo S, et al. Long‐term suppression of endometriosis associated pelvic pain with sequential administration of a gonadotropin releasing hormone agonist followed by danazol, oral contraceptives or dienogest. Journal of Endometriosis 2012;4(3):150.
Long 2010 {published data only}
-
- Long QQ, Zhang SF, Han Y, Chen H, Li XL, Hua KQ, et al. Clinical efficacy and safety of gonadotropin releasing hormone agonist combined with estrogen‐dydrogesteronea in treatment of endometriosis. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2010;45(4):247‐51. - PubMed
Moawad 2012 {published data only}
-
- Moawad A, Salah A, Abou‐Ria H, Abd‐Elzaher M, Madkour W. Is continuous use of long term OCP's more significant than cyclic use in prevention of endometrioma recurrence after laparoscopic excision? A randomized controlled trial. Human Reproduction 2012;27(Suppl 2):ii66‐7.
Muzii 2011 {published data only}
-
- Muzii L, Maneschi F, Marana R, Porpora MG, Zupi E, Bellati F, et al. Oral estroprogestins after laparoscopic surgery to excise endometriomas: continuous or cyclic administration? Results of a multicenter randomized study. Journal of Minimally Invasive Gynecology 2011;18(2):173‐8. - PubMed
Portman 2011 {published data only}
-
- Portman D, Reape K, Hait H, Howard B. Reduction in dysmenorrhea severity in women using a 91‐day extended regimen oral contraceptive compared to a 28‐day regimen oral contraceptive for the treatment of cyclic pelvic pain. Fertility and Sterility 2011;96(3 Suppl):S110.
Seracchioli 2010 {published data only}
-
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Montanari G, Keramyda A, et al. Long‐term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertility and Sterility 2010;93(1):52‐6. - PubMed
-
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Savelli L, Venturoli S. Long‐term oral contraceptive pills and postoperative pain management after laparoscopic excision of ovarian endometrioma: a randomized controlled trial. Fertility and Sterility 2010;94(2):464‐71. - PubMed
Sesti 2007 {published data only}
-
- Sesti F, Pietropolli A, Capozzolo T, Broccoli P, Pierangeli S, Bollea MR, et al. Hormonal suppression treatment or dietary therapy versus placebo in the control of painful symptoms after conservative surgery for endometriosis stage III‐IV. A randomized comparative trial. Fertility and Sterility 2007;88(6):1541‐7. - PubMed
Shturkalev 1970 {published data only}
-
- Shturkalev I, Katsulov A. Results of clinical trials of Ovostat as a contraceptive and therapeutic agent. Akusherstvo i Ginekologiia 1970;9(5):383‐7. - PubMed
Strowitzki 2012 {published data only}
-
- Strowitzki T, Kirsch B, Elliesen J. Efficacy of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen in women with moderate‐to‐severe primary dysmenorrhoea: An open‐label, multicentre, randomised, controlled study. Journal of Family Planning and Reproductive Health Care 2012;38(2):94‐101. - PMC - PubMed
Tanaka 2016 {published data only}
-
- Tanaka Y, Mori T, Ito F, Koshiba A, Kusuki I, Kitawaki J. Effects of low‐dose combined drospirenone‐ethinylestradiol on perimenstrual symptoms experienced by women with endometriosis. International Journal of Gynecology and Obstetrics 2016;135(2):135‐9. - PubMed
Taniguchi 2015 {published data only}
-
- Taniguchi F, Enatsu A, Ota I, Toda T, Arata K, Harada T. Effects of low dose oral contraceptive pill containing drospirenone/ethinylestradiol in patients with endometrioma. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;191:116‐20. - PubMed
Vercellini 2002 {published data only}
-
- Vercellini P, Giorgi O, Mosconi P, Stellato G, Vicentini S, Crosignani PG. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertility and Sterility 2002;77(1):52‐61. - PubMed
Vercellini 2005 {published data only}
-
- Vercellini P, Pietropaolo G, Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen‐progestogen combination versus low‐dose norethindrone acetate. Fertility and Sterility 2005;84(5):1375‐87. - PubMed
Xu 2011 {published data only}
-
- Xu XW, Wang LD, Zhu XQ, Yan LZ, Guan YT, Zhu SC, et al. Levonorgestrel‐releasing intrauterine system and combined oral contraceptives as conservative treatments for recurrent ovarian endometriosis: a comparative clinical study. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2011;91(15):1047‐50. - PubMed
Zhu 2014 {published data only}
-
- Zhu S, Liu D, Huang W, Wang Q, Zhou L, Feng G. Post‐laparoscopic oral contraceptive combined with Chinese herbal mixture in treatment of infertility and pain associated with minimal or mild endometriosis: a randomized controlled trial. BMC Complementary and Alternative Medicine 2014;14(1):222. - PMC - PubMed
Additional references
Ahangari 2014
-
- Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician 2014;17(2):E141‐7. - PubMed
Al‐Jefout 2011
-
- Al‐Jefout M. Brief update on endometriosis treatment. Middle East Fertility Society Journal 2011;16(3):167‐74.
ASRM 1997
-
- American Society of Reproductive Medicine. Revised American Society of Reproductive Medicine Classification of Endometriosis: 1996. Fertility and Sterility 1997;67(5):817‐21. - PubMed
Barlow 2005
-
- Barlow DH, Kennedy S. Endometriosis: new genetic approaches and therapy. Annual Review of Medicine 2005;56:345‐56. - PubMed
Bateson 2016
-
- Bateson D, Butcher BE, Donovan C, Farrell L, Kovacs G, Mezzini T, et al. Risk of venous thromboembolism in women taking the combined oral contraceptive: a systematic review and meta‐analysis. Australian Family Physician 2016;45(1‐2):59‐64. - PubMed
Biberoglu 1981
-
- Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short‐term and long‐term effectiveness. American Journal of Obstetrics and Gynecology 1981;139:645. - PubMed
Crosignani 2006
-
- Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Human Reproduction Update 2006;12(2):179‐89. - PubMed
Dunselman 2014
-
- Dunselman GA, Vermeulen N, Becker C, Calhaz‐Jorge C, D'Hooghe T, Bie B, et al. European Society of Human Reproduction Embryology. ESHRE guideline: management of women with endometriosis. Human Reproduction 2014;29(3):400‐12. - PubMed
Fauconnier 2005
-
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and its implications. Human Reproduction Update 2005;11(5):595‐606. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADE Working Group, McMaster. GRADEpro GDT. Version accessed 10 November 2017. Hamilton (ON): GRADE Working Group, McMaster, 2015. Available at gradepro.org.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jacobson 2002
Johnson 2006
-
- Johnson N, Farquhar C. Endometriosis. Clinical Evidence 2006;15:2449‐64. - PubMed
Jones 2004
-
- Jones G, Jenkinson C, Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. Journal of Psychosomatic Obstetrics and Gynaecology 2004;25:123‐33. - PubMed
Kennedy 2006
-
- Kennedy 2000 on behalf of the Guidelines and Audit committee of the Royal College of Obstetricians and Gynaecologist. The investigation and management of endometriosis 24. Royal College of Obstetricians and Gynaecologists: Green Top Guidelines 2006.
Kistner 1958
-
- Kistner RW. The use of newer progestins in the treatment of endometriosis. American Journal of Obstetrics and Gynecology 1958;75:264‐78. - PubMed
Kistner 1959
-
- Kistner RW. Treatment of endometriosis by inducing pseudo‐pregnancy with ovarian hormones. Fertility and Sterility 1959;10:539‐54.
Lancaster 1995
-
- Lancaster JM, Prentice A, Smith SK. Successful medical treatment of sub‐diaphragmatic endometriosis. Journal of Obstetrics and Gynaecology 1995;15:206‐9.
Meresman 2002
-
- Meresman GF, Auge L, Baranoa RI, Lombardi E, Tesone M, Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of ectopic endometrial tissue from patients with endometriosis. Fertility and Sterility 2002;77(6):1141‐7. - PubMed
Vessey 1993
Yap 2004
References to other published versions of this review
Davis 1997
-
- Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD001019] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

