Microtubule Dynamics Scale with Cell Size to Set Spindle Length and Assembly Timing
- PMID: 29787710
- PMCID: PMC6360954
- DOI: 10.1016/j.devcel.2018.04.022
Microtubule Dynamics Scale with Cell Size to Set Spindle Length and Assembly Timing
Abstract
Successive cell divisions during embryonic cleavage create increasingly smaller cells, so intracellular structures must adapt accordingly. Mitotic spindle size correlates with cell size, but the mechanisms for this scaling remain unclear. Using live cell imaging, we analyzed spindle scaling during embryo cleavage in the nematode Caenorhabditis elegans and sea urchin Paracentrotus lividus. We reveal a common scaling mechanism, where the growth rate of spindle microtubules scales with cell volume, which explains spindle shortening. Spindle assembly timing is, however, constant throughout successive divisions. Analyses in silico suggest that controlling the microtubule growth rate is sufficient to scale spindle length and maintain a constant assembly timing. We tested our in silico predictions to demonstrate that modulating cell volume or microtubule growth rate in vivo induces a proportional spindle size change. Our results suggest that scalability of the microtubule growth rate when cell size varies adapts spindle length to cell volume.
Keywords: Caenorhabditis elegans; Paracentrotus lividus; cell division; embryonic development; in silico models; intracellular scaling; microtubule dynamics; microtubules; mitotic spindle; spindle assembly.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures


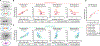



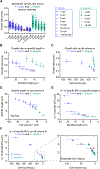

Comment in
-
The Incredible Shrinking Spindle.Dev Cell. 2018 May 21;45(4):421-423. doi: 10.1016/j.devcel.2018.05.007. Dev Cell. 2018. PMID: 29787705
References
-
- Agrell I (1956). A mitotic gradient as the cause of the early differentiation in the sea urchin embryo. Zoological Papers in Honour of B Hanstrom, pp. 27–34.
-
- Andersen SS (2000). Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol 10, 261–267. - PubMed
-
- Andersen SS, Ashford AJ, Tournebize R, Gavet O, Sobel A, Hyman AA, and Karsenti E (1997). Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature 389, 640–643. - PubMed
-
- Angerer LM, and Angerer RC (2000). Animal-vegetal axis patterning mechanisms in the early sea urchin embryo. Dev Biol 218, 1–12. - PubMed
-
- Belmont LD, Hyman AA, Sawin KE, and Mitchison TJ (1990). Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 62, 579–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

