Conservative Management Following Complete Clinical Response to Neoadjuvant Chemotherapy of Muscle Invasive Bladder Cancer: Contemporary Outcomes of a Multi-Institutional Cohort Study
- PMID: 29787740
- PMCID: PMC7543664
- DOI: 10.1016/j.juro.2018.05.078
Conservative Management Following Complete Clinical Response to Neoadjuvant Chemotherapy of Muscle Invasive Bladder Cancer: Contemporary Outcomes of a Multi-Institutional Cohort Study
Abstract
Purpose: We report the outcomes in patients with muscle invasive bladder cancer from 2 institutions who experienced a clinically complete response to neoadjuvant platinum based chemotherapy and elected active surveillance. It was unknown whether conservative treatment could be safely implemented in these patients.
Materials and methods: We retrospectively reviewed the records of patients with muscle invasive bladder cancer at our institutions who elected surveillance following a clinically complete response to transurethral resection of bladder tumors and neoadjuvant chemotherapy from 2001 to 2017. A clinically complete response was defined as absent tumor on post-chemotherapy transurethral resection of bladder tumor, negative cytology and normal cross-sectional imaging.
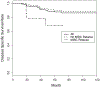
Results: In the 148 patients followed a median of 55 months (range 5 to 145) the 5-year disease specific, overall, cystectomy-free and recurrence-free survival rates were 90%, 86%, 76% and 64%, respectively. Of the patients 71 (48%) experienced recurrence in the bladder, including 16 (11%) with muscle invasive disease and 55 (37%) with noninvasive disease. Salvage radical cystectomy prevented cancer specific death in 9 of 12 patients (75%) who underwent cystectomy after muscle invasive relapse and in 13 of 14 (93%) after noninvasive relapse.
Conclusions: We observed high rates of overall and disease specific survival with bladder preservation in patients who achieved a clinically complete response to neoadjuvant chemotherapy. These outcomes support the safety of active surveillance in carefully selected, closely monitored patients with muscle invasive bladder cancer. Future studies should aim to improve patient selection by identifying biomarkers predicting invasive relapse and developing novel imaging methods of early detection.
Keywords: carcinoma; cystectomy; drug therapy; neoplasm invasiveness; urinary bladder neoplasms.
Copyright © 2018 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Editorial Comment.J Urol. 2018 Nov;200(5):1011-1012. doi: 10.1016/j.juro.2018.05.157. Epub 2018 Jul 29. J Urol. 2018. PMID: 30063891 No abstract available.
-
Editorial Comment.J Urol. 2018 Nov;200(5):1012. doi: 10.1016/j.juro.2018.05.158. Epub 2018 Jul 29. J Urol. 2018. PMID: 30063894 No abstract available.
-
Is it oncologically and clinically safe to adopt a bladder sparing approach in complete responders following neoadjuvant chemotherapy alone without proceeding to a radical cystectomy?Transl Androl Urol. 2018 Dec;7(Suppl 6):S741-S743. doi: 10.21037/tau.2018.08.18. Transl Androl Urol. 2018. PMID: 30687613 Free PMC article. No abstract available.
-
Re: Conservative Management Following Complete Clinical Response to Neoadjuvant Chemotherapy of Muscle Invasive Bladder Cancer: Contemporary Outcomes of a Multi-institutional Cohort Study.Eur Urol. 2019 Jul;76(1):127-129. doi: 10.1016/j.eururo.2019.03.001. Epub 2019 Mar 14. Eur Urol. 2019. PMID: 30879794 No abstract available.
References
-
- Siegel RL, Miller KD and Jemal A: Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7. - PubMed
-
- Burger M, Catto JW, Dalbagni G et al. : Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013; 63: 234. - PubMed
-
- Grossman HB, Natale RB, Tangen CM et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical