What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis
- PMID: 29788164
- PMCID: PMC5961275
- DOI: 10.1093/annonc/mdy071
What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis
Abstract
Background: Our prior Systemic Treatment Options for Cancer of the Prostate systematic reviews showed improved survival for men with metastatic hormone-naive prostate cancer when abiraterone acetate plus prednisolone/prednisone (AAP) or docetaxel (Doc), but not zoledronic acid (ZA), were added to androgen-deprivation therapy (ADT). Trial evidence also suggests a benefit of combining celecoxib (Cel) with ZA and ADT. To establish the optimal treatments, a network meta-analysis (NMA) was carried out based on aggregate data (AD) from all available studies.
Methods: Overall survival (OS) and failure-free survival data from completed Systemic Treatment Options for Cancer of the Prostate reviews of Doc, ZA and AAP and from recent trials of ZA and Cel contributed to this comprehensive AD-NMA. The primary outcome was OS. Correlations between treatment comparisons within one multi-arm, multi-stage trial were estimated from control-arm event counts. Network consistency and a common heterogeneity variance were assumed.
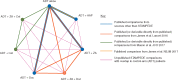
Results: We identified 10 completed trials which had closed to recruitment, and one trial in which recruitment was ongoing, as eligible for inclusion. Results are based on six trials including 6204 men (97% of men randomised in all completed trials). Network estimates of effects on OS were consistent with reported comparisons with ADT alone for AAP [hazard ration (HR) = 0.61, 95% confidence interval (CI) 0.53-0.71], Doc (HR = 0.77, 95% CI 0.68-0.87), ZA + Cel (HR = 0.78, 95% CI 0.62-0.97), ZA + Doc (HR = 0.79, 95% CI 0.66-0.94), Cel (HR = 0.94 95% CI 0.75-1.17) and ZA (HR = 0.90 95% CI 0.79-1.03). The effect of ZA + Cel is consistent with the additive effects of the individual treatments. Results suggest that AAP has the highest probability of being the most effective treatment both for OS (94% probability) and failure-free survival (100% probability). Doc was the second-best treatment of OS (35% probability).
Conclusions: Uniquely, we have included all available results and appropriately accounted for inclusion of multi-arm, multi-stage trials in this AD-NMA. Our results support the use of AAP or Doc with ADT in men with metastatic hormone-naive prostate cancer. AAP appears to be the most effective treatment, but it is not clear to what extent and whether this is due to a true increased benefit with AAP or the variable features of the individual trials. To fully account for patient variability across trials, changes in prognosis or treatment effects over time and the potential impact of treatment on progression, a network meta-analysis based on individual participant data is in development.
Figures



References
-
- White IR. Network meta-analysis. Stata J 2015; 15: 951–985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

