Monitoring Serious Adverse Events in the Sierra Leone Trial to Introduce a Vaccine Against Ebola
- PMID: 29788346
- PMCID: PMC5961367
- DOI: 10.1093/infdis/jiy042
Monitoring Serious Adverse Events in the Sierra Leone Trial to Introduce a Vaccine Against Ebola
Abstract
ClinicalTrials.gov [NCT02378753] and Pan African Clinical Trials Registry [PACTR201502001037220].
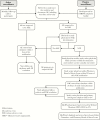
Figures
References
-
- World Health Organization (WHO). Ebola response roadmap update. Geneva: WHO, 2014.
-
- World Health Organization(WHO). Meeting summary of the WHO consultation on potential Ebola therapies and vaccines. Geneva: WHO, 2014.
-
- Widdowson MA, Schrag SJ, Carter RJ, et al. . Implementing an Ebola vaccine study —Sierra Leone. MMWR Suppl 2016; 65:98–106. - PubMed
-
- Guidance for Industry and Investigators. Safety reporting requirements for INDs and BA/BE studies. Silver Spring, MD: Food and Drug Administration, 2012.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical