Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer
- PMID: 29788892
- PMCID: PMC6110043
- DOI: 10.2174/1568026618666180523111351
Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer
Abstract
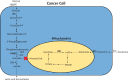
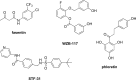
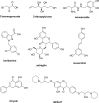
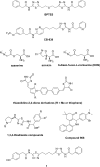
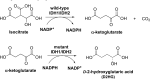
Cancer cells have a very different metabolism from that of normal cells from which they are derived. Their metabolism is elevated, which allows them to sustain higher proliferative rate and resist some cell death signals. This phenomenon, known as the "Warburg effect", has become the focus of intensive efforts in the discovery of new therapeutic targets and new cancer drugs. Both glycolysis and glutaminolysis pathways are enhanced in cancer cells. While glycolysis is enhanced to satisfy the increasing energy demand of cancer cells, glutaminolysis is enhanced to provide biosynthetic precursors for cancer cells. It was recently discovered that there is a tyrosine phosphorylation of a specific isoform of pyruvate kinase, the M2 isoform, that is preferentially expressed in all cancer cells, which results in the generation of pyruvate through a unique enzymatic mechanism that is uncoupled from ATP production. Pyruvate produced through this unique enzymatic mechanism is converted primarily into lactic acid, rather than acetyl-CoA for the synthesis of citrate, which would normally then enter the citric acid cycle. Inhibition of key enzymes in glycolysis and glutaminolysis pathways with small molecules has provided a novel but emerging area of cancer research and has been proven effective in slowing the proliferation of cancer cells, with several inhibitors being in clinical trials. This review paper will cover recent advances in the development of chemotherapeutic agents against several metabolic targets for cancer therapy, including glucose transporters, hexokinase, pyruvate kinase M2, glutaminase, and isocitrate dehydrogenase.
Keywords: Cancer; glucose transporters; glutaminase; glutaminolysis; glycolysis; hexokinase; isocitrate dehydrogenase; pyruvate kinase M2..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
Similar articles
-
Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression.Oncotarget. 2015 Sep 22;6(28):26161-76. doi: 10.18632/oncotarget.4544. Oncotarget. 2015. PMID: 26317652 Free PMC article.
-
Targeting cellular metabolism to improve cancer therapeutics.Cell Death Dis. 2013 Mar 7;4(3):e532. doi: 10.1038/cddis.2013.60. Cell Death Dis. 2013. PMID: 23470539 Free PMC article. Review.
-
Metabolic reprogramming in cancer cells: glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer.World J Surg Oncol. 2016 Jan 20;14(1):15. doi: 10.1186/s12957-016-0769-9. World J Surg Oncol. 2016. PMID: 26791262 Free PMC article. Review.
-
Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer.Semin Cell Dev Biol. 2020 Feb;98:34-43. doi: 10.1016/j.semcdb.2019.05.012. Epub 2019 May 22. Semin Cell Dev Biol. 2020. PMID: 31100352 Review.
-
Cancer cell metabolism: implications for therapeutic targets.Exp Mol Med. 2013 Oct 4;45(10):e45. doi: 10.1038/emm.2013.85. Exp Mol Med. 2013. PMID: 24091747 Free PMC article. Review.
Cited by
-
Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines.Metabolites. 2024 Aug 6;14(8):435. doi: 10.3390/metabo14080435. Metabolites. 2024. PMID: 39195531 Free PMC article.
-
Monitoring vascular normalization: new opportunities for mitochondrial inhibitors in breast cancer.Oncoscience. 2021 Feb 25;8:1-13. doi: 10.18632/oncoscience.523. eCollection 2021. Oncoscience. 2021. PMID: 33869665 Free PMC article.
-
O-GlcNAcylation: cellular physiology and therapeutic target for human diseases.MedComm (2020). 2023 Dec 19;4(6):e456. doi: 10.1002/mco2.456. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38116061 Free PMC article. Review.
-
The transcription factor ChREBP Orchestrates liver carcinogenesis by coordinating the PI3K/AKT signaling and cancer metabolism.Nat Commun. 2024 Feb 29;15(1):1879. doi: 10.1038/s41467-024-45548-w. Nat Commun. 2024. PMID: 38424041 Free PMC article.
-
Inhibition of cancer metabolism: a patent landscape.Pharm Pat Anal. 2019 Jul;8(4):117-138. doi: 10.4155/ppa-2019-0012. Epub 2019 Aug 15. Pharm Pat Anal. 2019. PMID: 31414969 Free PMC article. Review.
References
-
- Stewart B.W., Wild C.P. World Cancer Rep., 2014. IARC; 2014.
-
- Tennant D.A., Durán R.V., Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer. 2010;10:267–277. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Deng G., Shen J., Yin M., McManus J., Mathieu M., Gee P., He T., Shi C., Bedel O., McLean L.R., Le-Strat F., Zhang Y., Marquette J-P., Gao Q., Zhang B., Rak A., Hoffmann D., Rooney E., Vassort A., Englaro W., Li Y., Patel V., Adrian F., Gross S., Wiederschain D., Cheng H., Licht S. Selective inhibition of mutant isocitrate dehydrogenase 1 (IDH1) via disruption of a metal binding network by an allosteric small molecule. J. Biol. Chem. 2015;290:762–774. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

