Vaccines, inspiring innovation in health
- PMID: 29789241
- PMCID: PMC6238075
- DOI: 10.1016/j.vaccine.2018.05.035
Vaccines, inspiring innovation in health
Abstract
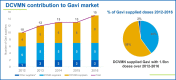
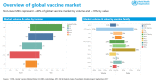
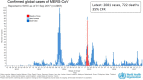
This report covers the topics of pandemics, epidemics and partnerships, including regulatory convergence initiatives, new technologies and novel vaccines, discussed by leading public and private sector stakeholders at the 18th Annual General Meeting (AGM) of the Developing Countries Vaccine Manufacturers' Network (DCVMN). Contributions of Gavi and the vaccine industry from emerging countries to the growing global vaccine market, by improving the supply base from manufacturers in developing countries and contributing to 58% of doses, were highlighted. The Coalition for Epidemic Preparedness Innovations (CEPI), the International Vaccine Institute (IVI) and others reported on new strategies to ensure speedy progress in preclinical and clinical development of innovative vaccines for future MERS, Zika or other outbreak response. Priorities for vaccine stockpiling, to assure readiness during emergencies and to prevent outbreaks due to re-emerging diseases such as yellow fever, cholera and poliomyelitis, were outlined. The role of partnerships in improving global vaccine access, procurement and immunization coverage, and shared concerns were reviewed. The World Health Organization (WHO) and other international collaborating partners provided updates on the Product, Price and Procurement database, the prequalification of vaccines, the control of neglected tropical diseases, particularly the new rabies elimination initiative, and regulatory convergence proposals to accelerate vaccine registration in developing countries. Updates on supply chain innovations and novel vaccine platforms were presented. The discussions enabled members and partners to reflect on efficiency of research & development, supply chain tools and trends in packaging technologies improving delivery of existing vaccines, and allowing a deeper understanding of the current public-health objectives, industry financing, and global policies, required to ensure optimal investments, alignment and stability of vaccine supply in developing countries.
Keywords: Developing countries; Epidemic; Procurement; Stockpiling; Technology; Vaccine manufacturing.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Kwon DC. Inaugral address: DCVMN Annual General Meeting. 2017. <http://www.dcvmn.org/2017-Annual-Meeting-in-Seoul>.
-
- Gavi. Annual progress report. 2016:65. <http://www.gavi.org/results/gavi-progress-reports/>.
-
- Stack B.M.L., Ozawa S., Bishai D.M., Mirelman A., Tam Y., Niessen L. Estimated economic benefits during the “ Decade Of Vaccines” include treatment savings, gains in labor productivity. Health Aff. 2011;30:1021–1028. - PubMed
-
- Lee L.A., Franzel L., Atwell J., Datta S.D., Friberg I.K., Goldie S.J. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine. 2013;315:B61–B72. - PubMed
-
- WHO. Progress and challenges with achieving universal immunization coverage: 2016 estimates of immunization coverage. 2017. <http://www.who.int/immunization/monitoring_surveillance/who-immuniz.pdf>.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

