Reactivation of γ-globin in adult β-YAC mice after ex vivo and in vivo hematopoietic stem cell genome editing
- PMID: 29789357
- PMCID: PMC6024639
- DOI: 10.1182/blood-2018-03-838540
Reactivation of γ-globin in adult β-YAC mice after ex vivo and in vivo hematopoietic stem cell genome editing
Abstract
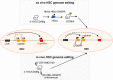
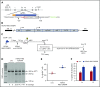
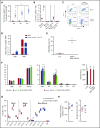
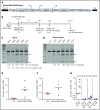
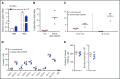
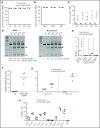
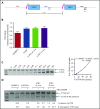
Disorders involving β-globin gene mutations, primarily β-thalassemia and sickle cell disease, represent a major target for hematopoietic stem/progenitor cell (HSPC) gene therapy. This includes CRISPR/Cas9-mediated genome editing approaches in adult CD34+ cells aimed toward the reactivation of fetal γ-globin expression in red blood cells. Because models involving erythroid differentiation of CD34+ cells have limitations in assessing γ-globin reactivation, we focused on human β-globin locus-transgenic (β-YAC) mice. We used a helper-dependent human CD46-targeting adenovirus vector expressing CRISPR/Cas9 (HDAd-HBG-CRISPR) to disrupt a repressor binding region within the γ-globin promoter. We transduced HSPCs from β-YAC/human CD46-transgenic mice ex vivo and subsequently transplanted them into irradiated recipients. Furthermore, we used an in vivo HSPC transduction approach that involves HSPC mobilization and the intravenous injection of HDAd-HBG-CRISPR into β-YAC/CD46-transgenic mice. In both models, we demonstrated efficient target site disruption, resulting in a pronounced switch from human β- to γ-globin expression in red blood cells of adult mice that was maintained after secondary transplantation of HSPCs. In long-term follow-up studies, we did not detect hematological abnormalities, indicating that HBG promoter editing does not negatively affect hematopoiesis. This is the first study that shows successful in vivo HSPC genome editing by CRISPR/Cas9.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Stamatoyannopolous G, Nienhuis AW. Hemoglobin switching. In: Stamatoyannopolous G, Nienhuis AW, Majerus PW, Varmus H, eds. The Molecular Basis of Blood Diseases. Philadelphia, PA: Saunders; 1994:107-155.
-
- Fraser P, Grosveld F. Locus control regions, chromatin activation and transcription. Curr Opin Cell Biol. 1998;10(3):361-365. - PubMed
-
- Bulger M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13(19):2465-2477. - PubMed
-
- Forget BG. Molecular basis of hereditary persistence of fetal hemoglobin. Ann N Y Acad Sci. 1998;850(1):38-44. - PubMed
-
- Vinjamur DS, Bauer DE, Orkin SH. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br J Haematol. 2018;180(5):630-643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

