Identification and Characterization of a Novel Spontaneously Active Bursty GABAergic Interneuron in the Mouse Striatum
- PMID: 29789374
- PMCID: PMC6010567
- DOI: 10.1523/JNEUROSCI.3354-17.2018
Identification and Characterization of a Novel Spontaneously Active Bursty GABAergic Interneuron in the Mouse Striatum
Abstract
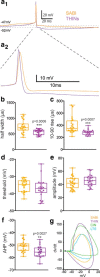
The recent availability of different transgenic mouse lines coupled with other modern molecular techniques has led to the discovery of an unexpectedly large cellular diversity and synaptic specificity in striatal interneuronal circuitry. Prior research has described three spontaneously active interneuron types in mouse striatal slices: the cholinergic interneuron, the neuropeptide Y-low threshold spike interneuron, and the tyrosine hydroxylase interneurons (THINs). Using transgenic Htr3a-Cre mice, we now characterize a fourth population of spontaneously active striatal GABAergic interneurons termed spontaneously active bursty interneurons (SABIs) because of their unique burst-firing pattern in cell-attached recordings. Although they bear some qualitative similarity in intrinsic electrophysiological properties to THINs in whole-cell recordings, detailed analysis revealed significant differences in many intrinsic properties and in their morphology. Furthermore, all previously identified striatal GABAergic interneurons have been shown to innervate striatal spiny projection neurons (SPNs), contributing to the suggestion that the principal function of striatal GABAergic interneurons is to provide feedforward inhibition to SPNs. Here, very surprisingly, paired recordings show that SABIs do not innervate SPNs significantly. Further, optogenetic inhibition of striatal Htr3a-Cre interneurons triggers barrages of IPSCs in SPNs. We hypothesize that these IPSCs result from disinhibition of a population of GABAergic interneurons with activity that is constitutively suppressed by the SABIs. We suggest that the SABIs represent the first example of a striatal interneuron-selective interneuron and, further, that their existence, along with previously defined interneuronal networks, may participate in the formation of SPN ensembles observed by others.SIGNIFICANCE STATEMENT Before ∼2010, the main function of the three known subtypes of striatal GABAergic interneurons was assumed to mediate feedforward inhibition of the spiny neurons (SPNs). During the past decade, we and others have described several novel populations of striatal GABAergic interneurons and their synaptic connections and have shown that striatal interneurons and SPNs interact through extensive and highly cell-type-specific connections that form specialized networks. Here, we describe a novel population of striatal GABAergic interneuron and provide several lines of evidence suggesting that it represents the first interneuron-selective interneuron in striatum. Striatal interneurons and their synaptic connections are suggested to play an important role in the formation of ensembles of striatal SPNs interconnected by inhibitory axon collaterals.
Keywords: basal ganglia; interneuron; interneuron-selective; network; optogenetic; striatum.
Copyright © 2018 the authors 0270-6474/18/385688-12$15.00/0.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
