Macrophage-Derived Granulin Drives Resistance to Immune Checkpoint Inhibition in Metastatic Pancreatic Cancer
- PMID: 29789416
- PMCID: PMC6076440
- DOI: 10.1158/0008-5472.CAN-17-3876
Macrophage-Derived Granulin Drives Resistance to Immune Checkpoint Inhibition in Metastatic Pancreatic Cancer
Abstract
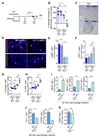
The ability of disseminated cancer cells to evade the immune response is a critical step for efficient metastatic progression. Protection against an immune attack is often provided by the tumor microenvironment that suppresses and excludes cytotoxic CD8+ T cells. Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive metastatic disease with unmet needs, yet the immunoprotective role of the metastatic tumor microenvironment in pancreatic cancer is not completely understood. In this study, we find that macrophage-derived granulin contributes to cytotoxic CD8+ T-cell exclusion in metastatic livers. Granulin expression by macrophages was induced in response to colony-stimulating factor 1. Genetic depletion of granulin reduced the formation of a fibrotic stroma, thereby allowing T-cell entry at the metastatic site. Although metastatic PDAC tumors are largely resistant to anti-PD-1 therapy, blockade of PD-1 in granulin-depleted tumors restored the antitumor immune defense and dramatically decreased metastatic tumor burden. These findings suggest that targeting granulin may serve as a potential therapeutic strategy to restore CD8+ T-cell infiltration in metastatic PDAC, thereby converting PDAC metastatic tumors, which are refractory to immune checkpoint inhibitors, into tumors that respond to immune checkpoint inhibition therapies.Significance: These findings uncover a mechanism by which metastatic PDAC tumors evade the immune response and provide the rationale for targeting granulin in combination with immune checkpoint inhibitors for the treatment of metastatic PDAC.Graphical Abstract: http://cancerres.aacrjournals.org/content/canres/78/15/4253/F1.large.jpg Cancer Res; 78(15); 4253-69. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures






References
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. - PubMed
-
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12(4):298–306. - PubMed
-
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–31. - PubMed
-
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. - PubMed
-
- Ribas A. Tumor immunotherapy directed at PD-1. The New England journal of medicine. 2012;366(26):2517–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

