Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer
- PMID: 29789717
- PMCID: PMC6137025
- DOI: 10.1038/s41388-018-0317-x
Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer
Abstract
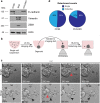
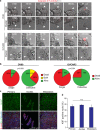
Ovarian cancer is the most lethal gynecological cancer, where survival rates have had modest improvement over the last 30 years. Metastasis of cancer cells is a major clinical problem, and patient mortality occurs when ovarian cancer cells spread beyond the confinement of ovaries. Disseminated ovarian cancer cells typically spread within the abdomen, where ascites accumulation aids in their transit. Metastatic ascites contain multicellular spheroids, which promote chemo-resistance and recurrence. However, little is known about the origin and mechanisms through which spheroids arise. Using live-imaging of 3D culture models and animal models, we report that epithelial ovarian cancer (EOC) cells, the most common type of ovarian cancer, can spontaneously detach as either single cells or clusters. We report that clusters are more resistant to anoikis and have a potent survival advantage over single cells. Using in vivo lineage tracing, we found that multicellular spheroids arise preferentially from collective detachment, rather than aggregation in the abdomen. Finally, we report that multicellular spheroids from collective detachment are capable of seeding intra-abdominal metastases that retain intra-tumoral heterogeneity from the primary tumor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

