Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy
- PMID: 29789983
- PMCID: PMC5964054
- DOI: 10.1186/s13613-018-0402-x
Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy
Abstract
In patients with septic shock, the administration of fluids during initial hemodynamic resuscitation remains a major therapeutic challenge. We are faced with many open questions regarding the type, dose and timing of intravenous fluid administration. There are only four major indications for intravenous fluid administration: aside from resuscitation, intravenous fluids have many other uses including maintenance and replacement of total body water and electrolytes, as carriers for medications and for parenteral nutrition. In this paradigm-shifting review, we discuss different fluid management strategies including early adequate goal-directed fluid management, late conservative fluid management and late goal-directed fluid removal. In addition, we expand on the concept of the "four D's" of fluid therapy, namely drug, dosing, duration and de-escalation. During the treatment of patients with septic shock, four phases of fluid therapy should be considered in order to provide answers to four basic questions. These four phases are the resuscitation phase, the optimization phase, the stabilization phase and the evacuation phase. The four questions are "When to start intravenous fluids?", "When to stop intravenous fluids?", "When to start de-resuscitation or active fluid removal?" and finally "When to stop de-resuscitation?" In analogy to the way we handle antibiotics in critically ill patients, it is time for fluid stewardship.
Keywords: Antibiotics; De-escalation; De-resuscitation; Dose; Drug; Duration; Fluid management; Fluid responsiveness; Fluid stewardship; Fluid therapy; Fluids; Four D’s; Four hits; Four indications; Four phases; Four questions; Goal-directed therapy; Maintenance; Monitoring; Passive leg raising; Replacement; Resuscitation.
Figures


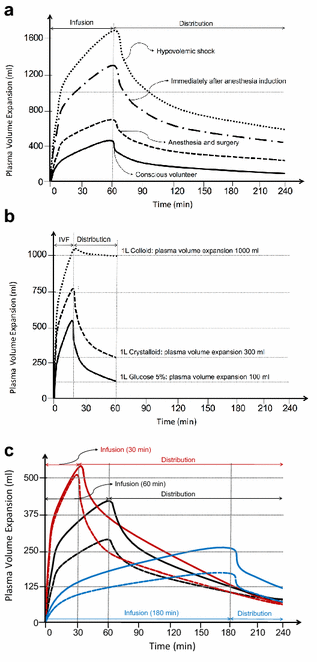
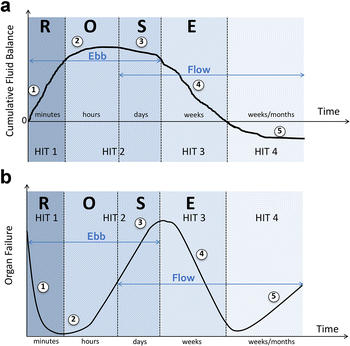

References
-
- Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–380. doi: 10.5603/AIT.2014.0060. - DOI - PubMed
-
- Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, Forceville X, Feissel M, Hasselmann M, Heininger A, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care. 2012;16(3):R94. doi: 10.1186/cc11358. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

