Development of an Electrochemical Paper-Based Analytical Device for Trace Detection of Virus Particles
- PMID: 29790331
- PMCID: PMC6595480
- DOI: 10.1021/acs.analchem.8b02042
Development of an Electrochemical Paper-Based Analytical Device for Trace Detection of Virus Particles
Abstract
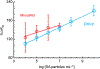
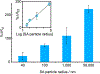
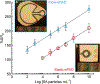
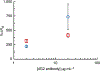
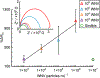
Viral pathogens are a serious health threat around the world, particularly in resource limited settings, where current sensing approaches are often insufficient and slow, compounding the spread and burden of these pathogens. Here, we describe a label-free, point-of-care approach toward detection of virus particles, based on a microfluidic paper-based analytical device with integrated microwire Au electrodes. The device is initially characterized through capturing of streptavidin modified nanoparticles by biotin-modified microwires. An order of magnitude improvement in detection limits is achieved through use of a microfluidic device over a classical static paper-based device, due to enhanced mass transport and capturing of particles on the modified electrodes. Electrochemical impedance spectroscopy detection of West Nile virus particles was carried out using antibody functionalized Au microwires, achieving a detection limit of 10.2 particles in 50 μL of cell culture media. No increase in signal is found on addition of an excess of a nonspecific target (Sindbis). This detection motif is significantly cheaper (∼$1 per test) and faster (∼30 min) than current methods, while achieving the desired selectivity and sensitivity. This sensing motif represents a general platform for trace detection of a wide range of biological pathogens.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

