Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes
- PMID: 29790784
- PMCID: PMC6290936
- DOI: 10.1164/rccm.201711-2287OC
Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes
Abstract
Rationale: Bronchopulmonary dysplasia (BPD) is a serious neonatal pulmonary condition associated with premature birth, but the underlying parenchymal disease and trajectory are poorly characterized. The current National Institute of Child Health and Human Development (NICHD)/NHLBI definition of BPD severity is based on degree of prematurity and extent of oxygen requirement. However, no clear link exists between initial diagnosis and clinical outcomes.
Objectives: We hypothesized that magnetic resonance imaging (MRI) of structural parenchymal abnormalities will correlate with NICHD-defined BPD disease severity and predict short-term respiratory outcomes.
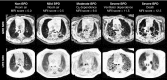
Methods: A total of 42 neonates (20 severe BPD, 6 moderate, 7 mild, 9 non-BPD control subjects; 40 ± 3-wk postmenstrual age) underwent quiet-breathing structural pulmonary MRI (ultrashort echo time and gradient echo) in a neonatal ICU-sited, neonatal-sized 1.5 T scanner, without sedation or respiratory support unless already clinically prescribed. Disease severity was scored independently by two radiologists. Mean scores were compared with clinical severity and short-term respiratory outcomes. Outcomes were predicted using univariate and multivariable models, including clinical data and scores.
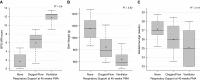
Measurements and main results: MRI scores significantly correlated with severities and predicted respiratory support at neonatal ICU discharge (P < 0.0001). In multivariable models, MRI scores were by far the strongest predictor of respiratory support duration over clinical data, including birth weight and gestational age. Notably, NICHD severity level was not predictive of discharge support.
Conclusions: Quiet-breathing neonatal pulmonary MRI can independently assess structural abnormalities of BPD, describe disease severity, and predict short-term outcomes more accurately than any individual standard clinical measure. Importantly, this nonionizing technique can be implemented to phenotype disease, and has potential to serially assess efficacy of individualized therapies.
Keywords: bronchopulmonary dysplasia; magnetic resonance imaging; neonatal lung disease; outcome prediction modeling; prematurity.
Figures



Comment in
-
Renewed Promise of Nonionizing Radiation Imaging for Chronic Lung Disease in Preterm Infants.Am J Respir Crit Care Med. 2018 Nov 15;198(10):1248-1249. doi: 10.1164/rccm.201805-0963ED. Am J Respir Crit Care Med. 2018. PMID: 29944841 No abstract available.
References
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. - PMC - PubMed
-
- Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013;37:132–137. - PubMed
-
- Gibson AM, Doyle LW. Respiratory outcomes for the tiniest or most immature infants. Semin Fetal Neonatal Med. 2014;19:105–111. - PubMed
-
- Simpson SJ, Hall GL, Wilson AC. Lung function following very preterm birth in the era of ‘new’ bronchopulmonary dysplasia. Respirology. 2015;20:535–540. - PubMed
-
- Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

