Nav1.7 is phosphorylated by Fyn tyrosine kinase which modulates channel expression and gating in a cell type-dependent manner
- PMID: 29790812
- PMCID: PMC6024516
- DOI: 10.1177/1744806918782229
Nav1.7 is phosphorylated by Fyn tyrosine kinase which modulates channel expression and gating in a cell type-dependent manner
Abstract
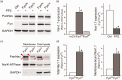
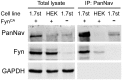
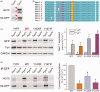
Voltage-gated sodium channel Nav1.7 is a key molecule in nociception, and its dysfunction has been associated with various pain disorders. Here, we investigated the regulation of Nav1.7 biophysical properties by Fyn, an Src family tyrosine kinase. Nav1.7 was coexpressed with either constitutively active (FynCA) or dominant negative (FynDN) variants of Fyn kinase. FynCA elevated protein expression and tyrosine phosphorylation of Nav1.7 channels. Site-directed mutagenesis analysis identified two tyrosine residues (Y1470 and Y1471) located within the Nav1.7 DIII-DIV linker (L3) as phosphorylation sites of Fyn. Whole-cell recordings revealed that FynCA evoked larger changes in Nav1.7 biophysical properties when expressed in ND7/23 cells than in Human Embryonic Kidney (HEK) 293 cells, suggesting a cell type-specific modulation of Nav1.7 by Fyn kinase. In HEK 293 cells, substitution of both tyrosine residues with phenylalanine dramatically reduced current amplitude of mutant channels, which was partially rescued by expressing mutant channels in ND7/23 cells. Phenylalanine substitution showed little effect on FynCA-induced changes in Nav1.7 activation and inactivation, suggesting additional modifications in the channel or modulation by interaction with extrinsic factor(s). Our study demonstrates that Nav1.7 is a substrate for Fyn kinase, and the effect of the channel phosphorylation depends on the cell background. Fyn-mediated modulation of Nav1.7 may regulate DRG neuron excitability and contribute to pain perception. Whether this interaction could serve as a target for developing new pain therapeutics requires future study.
Keywords: Fyn; Voltage-gated sodium channel; patch clamp; phosphorylation; tyrosine kinase.
Figures
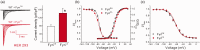


References
-
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The NaV1.7 sodium channel: from molecule to man. Nat Rev Neurosci 2013; 14: 49–62. - PubMed
-
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006; 444: 894–898. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

