Modulation of spinal motor networks by astrocyte-derived adenosine is dependent on D1-like dopamine receptor signaling
- PMID: 29790837
- PMCID: PMC6171060
- DOI: 10.1152/jn.00783.2017
Modulation of spinal motor networks by astrocyte-derived adenosine is dependent on D1-like dopamine receptor signaling
Abstract
Astrocytes modulate many neuronal networks, including spinal networks responsible for the generation of locomotor behavior. Astrocytic modulation of spinal motor circuits involves release of ATP from astrocytes, hydrolysis of ATP to adenosine, and subsequent activation of neuronal A1 adenosine receptors (A1Rs). The net effect of this pathway is a reduction in the frequency of locomotor-related activity. Recently, it was proposed that A1Rs modulate burst frequency by blocking the D1-like dopamine receptor (D1LR) signaling pathway; however, adenosine also modulates ventral horn circuits by dopamine-independent pathways. Here, we demonstrate that adenosine produced upon astrocytic stimulation modulates locomotor-related activity by counteracting the excitatory effects of D1LR signaling and does not act by previously described dopamine-independent pathways. In spinal cord preparations from postnatal mice, a D1LR agonist, SKF 38393, increased the frequency of locomotor-related bursting induced by 5-hydroxytryptamine and N-methyl-d-aspartate. Bath-applied adenosine reduced burst frequency only in the presence of SKF 38393, as did adenosine produced after activation of protease-activated receptor-1 to stimulate astrocytes. Furthermore, the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine enhanced burst frequency only in the presence of SKF 38393, indicating that endogenous adenosine produced by astrocytes during network activity also acts by modulating D1LR signaling. Finally, modulation of bursting by adenosine released upon stimulation of astrocytes was blocked by protein kinase inhibitor-(14-22) amide, a protein kinase A (PKA) inhibitor, consistent with A1R-mediated antagonism of the D1LR/adenylyl cyclase/PKA pathway. Together, these findings support a novel, astrocytic mechanism of metamodulation within the mammalian spinal cord, highlighting the complexity of the molecular interactions that specify motor output. NEW & NOTEWORTHY Astrocytes within the spinal cord produce adenosine during ongoing locomotor-related activity or when experimentally stimulated. Here, we show that adenosine derived from astrocytes acts at A1 receptors to inhibit a pathway by which D1-like receptors enhance the frequency of locomotor-related bursting. These data support a novel form of metamodulation within the mammalian spinal cord, enhancing our understanding of neuron-astrocyte interactions and their importance in shaping network activity.
Keywords: PAR1; central pattern generator; gliotransmission; metamodulation; motor control; neuromodulation.
Figures

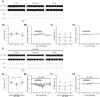


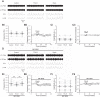

References
-
- Acevedo J, Santana-Almansa A, Matos-Vergara N, Marrero-Cordero LR, Cabezas-Bou E, Díaz-Ríos M. Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms. Neuropharmacology 101: 490–505, 2016. doi: 10.1016/j.neuropharm.2015.10.020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

