Reviewing the WHO guidelines for antibiotic use for sepsis in neonates and children
- PMID: 29790842
- PMCID: PMC6176768
- DOI: 10.1080/20469047.2017.1408738
Reviewing the WHO guidelines for antibiotic use for sepsis in neonates and children
Abstract
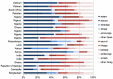
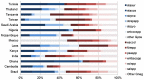
Background Guidelines from 2005 for treating suspected sepsis in low- and middle-income countries (LMIC) recommended hospitalisation and prophylactic intramuscular (IM) or intravenous (IV) ampicillin and gentamicin. In 2015, recommendations when referral to hospital is not possible suggest the administration of IM gentamicin and oral amoxicillin. In an era of increasing antimicrobial resistance, an updated review of the appropriate empirical therapy for treating sepsis (taking into account susceptibility patterns, cost and risk of adverse events) in neonates and children is necessary. Methods Systematic literature review and international guidelines were used to identify published evidence regarding the treatment of (suspected) sepsis. Results Five adequately designed and powered studies comparing antibiotic treatments in a low-risk community in neonates and young infants in LMIC were identified. These addressed potential simplifications of the current WHO treatment of reference, for infants for whom admission to inpatient care was not possible. Research is lacking regarding the treatment of suspected sepsis in neonates and children with hospital-acquired sepsis, despite rising antimicrobial resistance rates worldwide. Conclusions Current WHO guidelines supporting the use of gentamicin and penicillin for hospital-based patients or gentamicin (IM) and amoxicillin (oral) when referral to a hospital is not possible are in accordance with currently available evidence and other international guidelines, and there is no strong evidence to change this. The benefit of a cephalosporin alone or in combination as a second-line therapy in regions with known high rates of non-susceptibility is not well established. Further research into hospital-acquired sepsis in neonates and children is required.
Keywords: Sepsis; antibiotics; antimicrobial resistance; treatment guidelines.
Figures
References
-
- Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. - PubMed
-
- World Health Organization Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources; 2005. Available from: http://www.who.int/maternal_child_adolescent/documents/9241546700/en/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical