The way is the goal: how SecA transports proteins across the cytoplasmic membrane in bacteria
- PMID: 29790985
- PMCID: PMC5963308
- DOI: 10.1093/femsle/fny093
The way is the goal: how SecA transports proteins across the cytoplasmic membrane in bacteria
Abstract
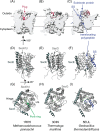
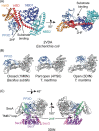
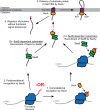
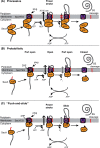
In bacteria, translocation of most soluble secreted proteins (and outer membrane proteins in Gram-negative bacteria) across the cytoplasmic membrane by the Sec machinery is mediated by the essential ATPase SecA. At its core, this machinery consists of SecA and the integral membrane proteins SecYEG, which form a protein conducting channel in the membrane. Proteins are recognised by the Sec machinery by virtue of an internally encoded targeting signal, which usually takes the form of an N-terminal signal sequence. In addition, substrate proteins must be maintained in an unfolded conformation in the cytoplasm, prior to translocation, in order to be competent for translocation through SecYEG. Recognition of substrate proteins occurs via SecA-either through direct recognition by SecA or through secondary recognition by a molecular chaperone that delivers proteins to SecA. Substrate proteins are then screened for the presence of a functional signal sequence by SecYEG. Proteins with functional signal sequences are translocated across the membrane in an ATP-dependent fashion. The current research investigating each of these steps is reviewed here.
Figures


References
-
- Akita M, Shinkai A, Matsuyama S et al. . SecA, an essential component of the secretory machinery of E. coli, exists as homodimer. Biochem Bioph Res Co 1991;174:211–6. - PubMed
-
- Andersson H, Bakker E, von Heijne G. Different positively charged amino acids have similar effects on the topology of a polytopic transmembrane protein in E. coli. J Biol Chem 1992;267:1491–5. - PubMed
-
- Antonoaea R, Furst M, Nishiyama K et al. . The periplasmic chaperone PpiD interacts with secretory proteins exiting from the SecYEG translocon. Biochemistry 2008;47:5649–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

