Reassessment of the Transport Mechanism of the Human Zinc Transporter SLC39A2
- PMID: 29791142
- PMCID: PMC7218795
- DOI: 10.1021/acs.biochem.8b00511
Reassessment of the Transport Mechanism of the Human Zinc Transporter SLC39A2
Abstract
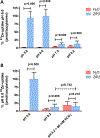
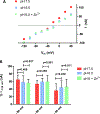
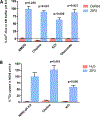
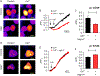
The human zinc transporter SLC39A2, also known as ZIP2, was shown to mediate zinc transport that could be inhibited at pH <7.0 and stimulated by HCO3-, suggesting a Zn2+/HCO3- cotransport mechanism [Gaither, L. A., and Eide, D. J. (2000) J. Biol. Chem. 275, 5560-5564]. In contrast, recent experiments in our laboratory indicated that the functional activity of ZIP2 increases at acidic pH [Franz, M. C., et al. (2014) J. Biomol. Screening 19, 909-916]. The study presented here was therefore designed to reexamine the findings about the pH dependence and to extend the functional characterization of ZIP2. Our current results show that ZIP2-mediated transport is modulated by extracellular pH but independent of the H+ driving force. Also, in our experiments, ZIP2-mediated transport is not modulated by extracellular HCO3-. Moreover, a high extracellular [K+], which induces depolarization, inhibited ZIP2-mediated transport, indicating that the transport mechanism is voltage-dependent. We also show that ZIP2 mediates the uptake of Cd2+ ( Km ∼ 1.57 μM) in a pH-dependent manner ( KH+ ∼ 66 nM). Cd2+ transport is inhibited by extracellular [Zn2+] (IC50 ∼ 0.32 μM), [Cu2+] (IC50 ∼ 1.81 μM), and to a lesser extent [Co2+], but not by [Mn2+] or [Ba2+]. Fe2+ is not transported by ZIP2. Accordingly, the substrate selectivity of ZIP2 decreases in the following order: Zn2+ > Cd2+ ≥ Cu2+ > Co2+. Altogether, we propose that ZIP2 is a facilitated divalent metal ion transporter that can be modulated by extracellular pH and membrane potential. Given that ZIP2 expression has been reported in acidic environments [Desouki, M. M., et al. (2007) Mol. Cancer 6, 37; Inoue, Y., et al. (2014) J. Biol. Chem. 289, 21451-21462; Tao, Y. T., et al. (2013) Mol. Biol. Rep. 40, 4979-4984], we suggest that the herein described H+-mediated regulatory mechanism might be important for determining the velocity and direction of the transport process.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Unraveling the structural elements of pH sensitivity and substrate binding in the human zinc transporter SLC39A2 (ZIP2).J Biol Chem. 2019 May 17;294(20):8046-8063. doi: 10.1074/jbc.RA118.006113. Epub 2019 Mar 26. J Biol Chem. 2019. PMID: 30914478 Free PMC article.
-
Structure, function, and regulation of a subfamily of mouse zinc transporter genes.J Biol Chem. 2003 Dec 12;278(50):50142-50. doi: 10.1074/jbc.M304163200. Epub 2003 Oct 2. J Biol Chem. 2003. PMID: 14525987
-
Development of the First Fluorescence Screening Assay for the SLC39A2 Zinc Transporter.J Biomol Screen. 2014 Jul;19(6):909-16. doi: 10.1177/1087057114526781. Epub 2014 Mar 11. J Biomol Screen. 2014. PMID: 24619115
-
Divalent metal transporter 1 in lead and cadmium transport.Ann N Y Acad Sci. 2004 Mar;1012:142-52. doi: 10.1196/annals.1306.011. Ann N Y Acad Sci. 2004. PMID: 15105261 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
Water molecules mediate zinc mobility in the bacterial zinc diffusion channel ZIPB.J Biol Chem. 2019 Sep 6;294(36):13327-13335. doi: 10.1074/jbc.RA119.009239. Epub 2019 Jul 18. J Biol Chem. 2019. PMID: 31320477 Free PMC article.
-
The Znt7-null mutation has sex dependent effects on the gut microbiota and goblet cell population in the mouse colon.PLoS One. 2020 Sep 29;15(9):e0239681. doi: 10.1371/journal.pone.0239681. eCollection 2020. PLoS One. 2020. PMID: 32991615 Free PMC article.
-
Structural insights into the elevator-type transport mechanism of a bacterial ZIP metal transporter.Nat Commun. 2023 Jan 24;14(1):385. doi: 10.1038/s41467-023-36048-4. Nat Commun. 2023. PMID: 36693843 Free PMC article.
-
Structural mechanism of intracellular autoregulation of zinc uptake in ZIP transporters.Nat Commun. 2023 Jun 9;14(1):3404. doi: 10.1038/s41467-023-39010-6. Nat Commun. 2023. PMID: 37296139 Free PMC article.
-
Structures, Mechanisms, and Physiological Functions of Zinc Transporters in Different Biological Kingdoms.Int J Mol Sci. 2024 Mar 6;25(5):3045. doi: 10.3390/ijms25053045. Int J Mol Sci. 2024. PMID: 38474291 Free PMC article. Review.
References
-
- Gaither LA, and Eide DJ (2000) Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem 275, 5560–5564. - PubMed
-
- Franz MC, Simonin A, Graeter S, Hediger MA, and Kovacs G (2014) Development of the First Fluorescence Screening Assay for the SLC39A2 Zinc Transporter. J. Biomol. Screening 19, 909–916. - PubMed
-
- Tao YT, Huang Q, Jiang YL, Wang XL, Sun P, Tian Y, Wu HL, Zhang M, Meng SB, Wang YS, Sun Q, and Zhang LY (2013) Up-regulation of Slc39A2(Zip2) mRNA in peripheral blood mononuclear cells from patients with pulmonary tuberculosis. Mol. Biol. Rep 40, 4979–4984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

