Role of adenosine signaling in coordinating cardiomyocyte function and coronary vascular growth in chronic fetal anemia
- PMID: 29791204
- PMCID: PMC6172624
- DOI: 10.1152/ajpregu.00319.2017
Role of adenosine signaling in coordinating cardiomyocyte function and coronary vascular growth in chronic fetal anemia
Abstract
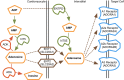
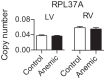
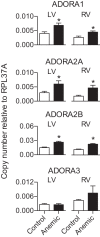
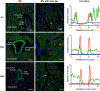
Fetal anemia causes rapid and profound changes in cardiac structure and function, stimulating proliferation of the cardiac myocytes, expansion of the coronary vascular tree, and impairing early contraction and relaxation. Although hypoxia-inducible factor-1α is sure to play a role, adenosine, a metabolic byproduct that increases coronary flow and growth, is implicated as a major stimulus for these adaptations. We hypothesized that genes involved in myocardial adenosine signaling would be upregulated in chronically anemic fetuses and that calcium-handling genes would be downregulated. After sterile surgical instrumentation under anesthesia, gestationally timed fetal sheep were made anemic by isovolumetric hemorrhage for 1 wk (16% vs. 35% hematocrit). At 87% of gestation, necropsy was performed to collect heart tissue for PCR and immunohistochemical analysis. Anemia increased mRNA expression levels of adenosine receptors ADORA 1, ADORA2A, and ADORA2B in the left and right ventricles (adenosine receptor ADORA3 was unchanged). In both ventricles, anemia also increased expression of ectonucleoside triphosphate diphosphohydrolase 1 and ecto-5'-nucleotidase. The genes for both equilibrative nucleoside transporters 1 and 2 were expressed more abundantly in the anemic right ventricle but were not different in the left ventricle. Neither adenosine deaminase nor adenosine kinase cardiac levels were significantly changed by chronic fetal anemia. Chronic fetal anemia did not significantly change cardiac mRNA expression levels of the voltage-dependent L-type calcium channel, ryanodine receptor 1, sodium-calcium exchanger, sarcoplasmic/endoplasmic reticulum calcium transporting ATPase 2, phospholamban, or cardiac calsequestrin. These data support local metabolic integration of vascular and myocyte function through adenosine signaling in the anemic fetal heart.
Keywords: adenosine signaling; fetal anemia; fetal heart development; programming.
Figures
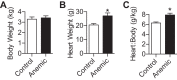


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

