Xenin is a novel anorexigen in goldfish (Carassius auratus)
- PMID: 29791497
- PMCID: PMC5965858
- DOI: 10.1371/journal.pone.0197817
Xenin is a novel anorexigen in goldfish (Carassius auratus)
Abstract
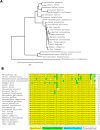
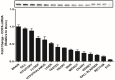
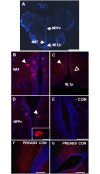
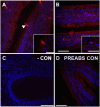
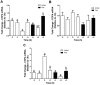
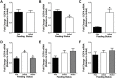
Xenin, a highly conserved 25 amino acid peptide cleaved from the N-terminus of the coatomer protein alpha (COPA), is emerging as a food intake regulator in mammals and birds. To date, no research has been conducted on xenin biology in fish. This study aims to identify the copa mRNA encoding xenin in goldfish (Carassius auratus) as a model, to elucidate its regulation by feeding, and to describe the role of xenin on appetite. First, a partial sequence of copa cDNA, a region encoding xenin, was identified from goldfish brain. This sequence is highly conserved among both vertebrates and invertebrates. RT-qPCR revealed that copa mRNAs are widely distributed in goldfish tissues, with the highest levels detected in the brain, gill, pituitary and J-loop. Immunohistochemistry confirmed also the presence of COPA peptide in the hypothalamus and enteroendocrine cells on the J-loop mucosa. In line with its anorexigenic effects, we found important periprandial fluctuations in copa mRNA expression in the hypothalamus, which were mainly characterized by a gradually decrease in copa mRNA levels as the feeding time was approached, and a gradual increase after feeding. Additionally, fasting differently modulated the expression of copa mRNA in a tissue-dependent manner. Peripheral and central injections of xenin reduce food intake in goldfish. This research provides the first report of xenin in fish, and shows that this peptide is a novel anorexigen in goldfish.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Feurle GE, Hamscher G, Kusiek R, Meyer HE, Metzger JW. Identification of xenin, a xenopsin-related peptide, in the human gastric mucosa and its effect on exocrine pancreatic secretion. J Biol Chem. 1992;267: 22305–22309. - PubMed
-
- Hamscher G, Meyer HE, Feurle GE. Identification of proxenin as a precursor of the peptide xenin with sequence homology to yeast and mammalian coat protein alpha. Peptides. 1996;17: 889–893. - PubMed
-
- Chow VT, Quek HH. Alpha coat protein COPA (HEP-COP): presence of an Alu repeat in cDNA and identity of the amino terminus to xenin. Ann Hum Genet. 1997;61: 369–373. doi: 10.1046/j.1469-1809.1997.6140369.x - DOI - PubMed
-
- Anlauf M, Weihe E, Hartschuh W, Hamscher G, Feurle GE. Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J Histochem Cytochem Off J Histochem Soc. 2000;48: 1617–1626. doi: 10.1177/002215540004801205 - DOI - PubMed
-
- Hamscher G, Meyer HE, Metzger JW, Feurle GE. Distribution, formation, and molecular forms of the peptide xenin in various mammals. Peptides. 1995;16: 791–797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

