[18F]FDG and [18F]FLT PET for the evaluation of response to neo-adjuvant chemotherapy in a model of triple negative breast cancer
- PMID: 29791503
- PMCID: PMC5965848
- DOI: 10.1371/journal.pone.0197754
[18F]FDG and [18F]FLT PET for the evaluation of response to neo-adjuvant chemotherapy in a model of triple negative breast cancer
Abstract
Rationale: Pathological response to neo-adjuvant chemotherapy (NAC) represents a commonly used predictor of survival in triple negative breast cancer (TNBC) and the need to identify markers that predict response to NAC is constantly increasing. Aim of this study was to evaluate the potential usefulness of PET imaging with [18F]FDG and [18F]FLT for the discrimination of TNBC responders to Paclitaxel (PTX) therapy compared to the response assessed by an adapted Response Evaluation Criteria In Solid Tumors (RECIST) criteria based on tumor volume (Tumor Volume Response).
Methods: Nu/nu mice bearing TNBC lesions of different size were evaluated with [18F]FDG and [18F]FLT PET before and after PTX treatment. SUVmax, Metabolic Tumor Volume (MTV) and Total Lesion Glycolysis (TLG) and Proliferation (TLP) were assessed using a graph-based random walk algorithm.
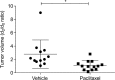
Results: We found that in our TNBC model the variation of [18F]FDG and [18F]FLT SUVmax similarly defined tumor response to therapy and that SUVmax variation represented the most accurate parameter. Response evaluation using Tumor Volume Response (TVR) showed that the effectiveness of NAC with PTX was completely independent from lesions size at baseline.
Conclusions: Our study provided interesting results in terms of sensitivity and specificity of PET in TNBC, revealing the similar performances of [18F]FDG and [18F]FLT in the identification of responders to Paclitaxel.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Le Du F, Eckhardt BL, Lim B, Litton JK, Moulder S, Meric-Bernstam F, et al. (2015) Is the future of personalized therapy in triple-negative breast cancer based on molecular subtype? Oncotarget 6: 12890–12908. doi: 10.18632/oncotarget.3849 - DOI - PMC - PubMed
-
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 26: 1275–1281. - PubMed
-
- von Minckwitz G (2012) Pathologic complete response after neoadjuvant therapy of breast cancer: pitfalls and shortcomings. Breast cancer research and treatment 132: 779–780. doi: 10.1007/s10549-012-2029-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

