PF-06526290 can both enhance and inhibit conduction through voltage-gated sodium channels
- PMID: 29791744
- PMCID: PMC6016627
- DOI: 10.1111/bph.14338
PF-06526290 can both enhance and inhibit conduction through voltage-gated sodium channels
Abstract
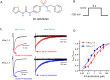
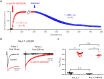
Background and purpose: Pharmacological agents that either inhibit or enhance flux of ions through voltage-gated sodium (Nav ) channels may provide opportunities for treatment of human health disorders. During studies to characterize agents that modulate Nav 1.3 function, we identified a compound that appears to exhibit both enhancement and inhibition of sodium ion conduction that appeared to be dependent on the gating state that the channel was in. The objective of the current study was to determine if these different modulatory effects are mediated by the same or distinct interactions with the channel.
Experimental approach: Electrophysiology and site-directed mutation were used to investigate the effects of PF-06526290 on Nav channel function.
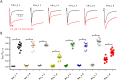
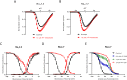
Key results: PF-06526290 greatly slows inactivation of Nav channels in a subtype-independent manner. However, upon prolonged depolarization to induce inactivation, PF-06526290 becomes a Nav subtype-selective inhibitor. Mutation of the domain 4 voltage sensor modulates inhibition of Nav 1.3 or Nav 1.7 channels by PF-06526290 but has no effect on PF-06526290 mediated slowing of inactivation.
Conclusions and implications: These findings suggest that distinct interactions may underlie the two modes of Nav channel modulation by PF-06526290 and that a single compound can affect sodium channel function in several ways.
© 2018 The British Pharmacological Society.
Figures



References
-
- Abbas N, Gaudioso‐Tyzra C, Bonnet C, Gabriac M, Amsalem M, Lonigro A et al (2013). The scorpion toxin Amm VIII induces pain hypersensitivity through gain‐of‐function of TTX‐sensitive Na(+) channels. Pain 154: 1204–1215. - PubMed
-
- Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H et al (2015). Structural basis of Nav1.7 inhibition by an isoform‐selective small‐molecule antagonist. Science 350: aac5464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

