A Multi-layered Quantitative In Vivo Expression Atlas of the Podocyte Unravels Kidney Disease Candidate Genes
- PMID: 29791858
- PMCID: PMC5986710
- DOI: 10.1016/j.celrep.2018.04.059
A Multi-layered Quantitative In Vivo Expression Atlas of the Podocyte Unravels Kidney Disease Candidate Genes
Abstract
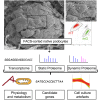
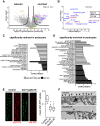
Damage to and loss of glomerular podocytes has been identified as the culprit lesion in progressive kidney diseases. Here, we combine mass spectrometry-based proteomics with mRNA sequencing, bioinformatics, and hypothesis-driven studies to provide a comprehensive and quantitative map of mammalian podocytes that identifies unanticipated signaling pathways. Comparison of the in vivo datasets with proteomics data from podocyte cell cultures showed a limited value of available cell culture models. Moreover, in vivo stable isotope labeling by amino acids uncovered surprisingly rapid synthesis of mitochondrial proteins under steady-state conditions that was perturbed under autophagy-deficient, disease-susceptible conditions. Integration of acquired omics dimensions suggested FARP1 as a candidate essential for podocyte function, which could be substantiated by genetic analysis in humans and knockdown experiments in zebrafish. This work exemplifies how the integration of multi-omics datasets can identify a framework of cell-type-specific features relevant for organ health and disease.
Keywords: end-stage renal disease; focal segmental glomerulosclerosis; hereditary nephrotic syndrome; kinase; metabolism; proteinuria; proteostasis; pulse SILAC; slit diaphragm; systems biology.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Quantitative deep mapping of the cultured podocyte proteome uncovers shifts in proteostatic mechanisms during differentiation.Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C404-17. doi: 10.1152/ajpcell.00121.2016. Epub 2016 Jun 29. Am J Physiol Cell Physiol. 2016. PMID: 27357545
-
Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease.Am J Pathol. 2013 Apr;182(4):1196-204. doi: 10.1016/j.ajpath.2012.12.011. Epub 2013 Feb 4. Am J Pathol. 2013. PMID: 23385000
-
Dosage-dependent role of Rac1 in podocyte injury.Am J Physiol Renal Physiol. 2016 Apr 15;310(8):F777-F784. doi: 10.1152/ajprenal.00381.2015. Epub 2016 Jan 20. Am J Physiol Renal Physiol. 2016. PMID: 26792065 Free PMC article.
-
Podocytes and the quest for precision medicines for kidney diseases.Pflugers Arch. 2017 Aug;469(7-8):1029-1037. doi: 10.1007/s00424-017-2015-x. Epub 2017 Jun 23. Pflugers Arch. 2017. PMID: 28646406 Review.
-
Targeting podocyte-associated diseases.Adv Drug Deliv Rev. 2010 Nov 30;62(14):1325-36. doi: 10.1016/j.addr.2010.08.012. Epub 2010 Sep 7. Adv Drug Deliv Rev. 2010. PMID: 20828590 Review.
Cited by
-
Human pluripotent stem cell-derived kidney organoids for personalized congenital and idiopathic nephrotic syndrome modeling.Development. 2022 May 1;149(9):dev200198. doi: 10.1242/dev.200198. Epub 2022 May 6. Development. 2022. PMID: 35417019 Free PMC article.
-
DACH1 as a multifaceted and potentially druggable susceptibility factor for kidney disease.J Clin Invest. 2021 May 17;131(10):e149043. doi: 10.1172/JCI149043. J Clin Invest. 2021. PMID: 33998596 Free PMC article.
-
The tissue proteome in the multi-omic landscape of kidney disease.Nat Rev Nephrol. 2021 Mar;17(3):205-219. doi: 10.1038/s41581-020-00348-5. Epub 2020 Oct 7. Nat Rev Nephrol. 2021. PMID: 33028957 Review.
-
Real-time imaging of cGMP signaling shows pronounced differences between glomerular endothelial cells and podocytes.Sci Rep. 2024 Oct 30;14(1):26099. doi: 10.1038/s41598-024-76768-1. Sci Rep. 2024. PMID: 39478086 Free PMC article.
-
Perspectives in systems nephrology.Cell Tissue Res. 2021 Aug;385(2):475-488. doi: 10.1007/s00441-021-03470-3. Epub 2021 May 24. Cell Tissue Res. 2021. PMID: 34027630 Free PMC article. Review.
References
-
- Bartram M.P., Habbig S., Pahmeyer C., Höhne M., Weber L.T., Thiele H., Altmüller J., Kottoor N., Wenzel A., Krueger M. Three-layered proteomic characterization of a novel ACTN4 mutation unravels its pathogenic potential in FSGS. Hum. Mol. Genet. 2016;25:1152–1164. - PubMed
-
- Boerries M., Grahammer F., Eiselein S., Buck M., Meyer C., Goedel M., Bechtel W., Zschiedrich S., Pfeifer D., Laloë D. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 2013;83:1052–1064. - PubMed
-
- Boute N., Gribouval O., Roselli S., Benessy F., Lee H., Fuchshuber A., Dahan K., Gubler M.C., Niaudet P., Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 2000;24:349–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

