Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies
- PMID: 29792024
- PMCID: PMC6192262
- DOI: 10.1021/acschemneuro.8b00200
Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies
Abstract
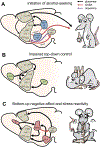
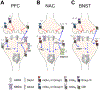
Developing efficacious treatments for alcohol use disorder (AUD) has proven difficult. The insidious nature of the disease necessitates a deep understanding of its underlying biology as well as innovative approaches to ameliorate ethanol-related pathophysiology. Excessive ethanol seeking and relapse are generated by long-term changes to membrane properties, synaptic physiology, and plasticity throughout the limbic system and associated brain structures. Each of these factors can be modulated by metabotropic glutamate (mGlu) receptors, a diverse set of G protein-coupled receptors highly expressed throughout the central nervous system. Here, we discuss how different components of the mGlu receptor family modulate neurotransmission in the limbic system and other brain regions involved in AUD etiology. We then describe how these processes are dysregulated following ethanol exposure and speculate about how mGlu receptor modulation might restore such pathophysiological changes. To that end, we detail the current understanding of the behavioral pharmacology of mGlu receptor-directed drug-like molecules in animal models of AUD. Together, this review highlights the prominent position of the mGlu receptor system in the pathophysiology of AUD and provides encouragement that several classes of mGlu receptor modulators may be translated as viable treatment options.
Keywords: Alcohol use disorder; bed nucleus of the stria terminalis; metabotropic glutamate receptor; nucleus accumbens; prefrontal cortex; synaptic plasticity.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.J.C. has been funded by the National Institutes of Health, AstraZeneca, Bristol-Myers Squibb, the Michael J. Fox Foundation, the Dystonia Medical Research Foundation, the CHDI Foundation, and the Thome Memorial Foundation. Over the past three years, he has served on the Scientific Advisory Boards for the Michael J. Fox Foundation, the Stanley Center for Psychiatric Research Broad Institute, Karuna Pharmaceuticals, the Lieber Institute for Brain Development, Clinical Mechanism and Proof of Concept Consortium, and the Neurobiology Foundation for Schizophrenia and Bipolar Disorder. P.J.C. is an inventor on patents that protect different classes of mGlu receptor allosteric modulators. M.E.J., S.W.C., A.A.J., and D.G.W. declare no conflicts of interest.
Figures
Similar articles
-
Sex differences and hormonal regulation of metabotropic glutamate receptor synaptic plasticity.Int Rev Neurobiol. 2023;168:311-347. doi: 10.1016/bs.irn.2022.10.002. Epub 2022 Nov 11. Int Rev Neurobiol. 2023. PMID: 36868632 Free PMC article. Review.
-
Allosteric modulation of metabotropic glutamate receptors in alcohol use disorder: Insights from preclinical investigations.Adv Pharmacol. 2020;88:193-232. doi: 10.1016/bs.apha.2020.02.002. Epub 2020 Mar 2. Adv Pharmacol. 2020. PMID: 32416868 Free PMC article. Review.
-
Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala.J Neurosci. 2006 Sep 27;26(39):9967-74. doi: 10.1523/JNEUROSCI.2384-06.2006. J Neurosci. 2006. PMID: 17005860 Free PMC article.
-
Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors.J Neurosci. 2016 Nov 9;36(45):11521-11531. doi: 10.1523/JNEUROSCI.1519-16.2016. J Neurosci. 2016. PMID: 27911756 Free PMC article.
-
Group III metabotropic glutamate receptors: pharmacology, physiology and therapeutic potential.Neurochem Res. 2014 Oct;39(10):1876-94. doi: 10.1007/s11064-014-1415-y. Epub 2014 Aug 22. Neurochem Res. 2014. PMID: 25146900 Review.
Cited by
-
Pleiotropic Effects of Grm7/GRM7 in Shaping Neurodevelopmental Pathways and the Neural Substrate of Complex Behaviors and Disorders.Biomolecules. 2025 Mar 8;15(3):392. doi: 10.3390/biom15030392. Biomolecules. 2025. PMID: 40149928 Free PMC article. Review.
-
Sex differences and hormonal regulation of metabotropic glutamate receptor synaptic plasticity.Int Rev Neurobiol. 2023;168:311-347. doi: 10.1016/bs.irn.2022.10.002. Epub 2022 Nov 11. Int Rev Neurobiol. 2023. PMID: 36868632 Free PMC article. Review.
-
Allosteric modulation of metabotropic glutamate receptors in alcohol use disorder: Insights from preclinical investigations.Adv Pharmacol. 2020;88:193-232. doi: 10.1016/bs.apha.2020.02.002. Epub 2020 Mar 2. Adv Pharmacol. 2020. PMID: 32416868 Free PMC article. Review.
-
mGlu2 and mGlu3 receptor negative allosteric modulators attenuate the interoceptive effects of alcohol in male and female rats.Pharmacol Biochem Behav. 2024 Jun;239:173767. doi: 10.1016/j.pbb.2024.173767. Epub 2024 Apr 10. Pharmacol Biochem Behav. 2024. PMID: 38608960 Free PMC article.
-
Targeting Neuroplasticity in Substance Use Disorders: Implications for Therapeutics.Annu Rev Pharmacol Toxicol. 2025 Jan;65(1):259-280. doi: 10.1146/annurev-pharmtox-061724-080548. Epub 2024 Dec 17. Annu Rev Pharmacol Toxicol. 2025. PMID: 39374445 Review.
References
-
- Koob GF, and Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

