Listeria monocytogenes infection enhances the interaction between rat non-classical MHC-Ib molecule and Ly49 receptors
- PMID: 29792127
- PMCID: PMC6830922
- DOI: 10.1177/1753425918759589
Listeria monocytogenes infection enhances the interaction between rat non-classical MHC-Ib molecule and Ly49 receptors
Abstract
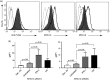
Murine NK cell Ly49 receptors, functionally analogous to KIRs in humans recognize MHC class I molecules and play a key role in controlling NK cell function. We have previously shown that the paired activating Ly49s4 and inhibitory Ly49i4 receptors recognize undefined non-classical MHC-Ib ligands from the RT1-CE region in rats. Here, the RT1-CE16 gene of the RT1d haplotype was stably transfected into the mouse RAW macrophage cell line, termed RAW-CE16d cells. Combining RAW-CE16d cells with Ly49 expressing reporter cells demonstrated Ly49i4 and Ly49s4 specificity for CE16d. The Ly49s4/i4:CE16d interaction was confirmed by specific MHC-I blocking monoclonal Abs. Further, we used our in vitro model to study the effect of Listeria monocytogenes (LM) on CE16d after infection. LM infection and IFN-γ stimulation both led to enhanced CE16d expression on the surface of transfected RAW-CE16d cells. Interestingly, the reporter cells displayed increased response to LM-infected RAW-CE16d cells compared with IFN-γ-treated RAW-CE16d cells, suggesting a fundamental difference between these stimuli in supporting enhanced Ly49 recognition of CE16d. Collectively, our data show that Ly49s4 and Ly49i4 recognize the non-classical RT1-CE16d molecule, which in turn is up-regulated during LM infection and thereby may contribute to NK-mediated responses against infected cells.
Keywords: CE16 molecule; Listeria monocytogenes; Ly49; MHC-Ib; NK cells; RT1-CEd.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Travier L, Lecuit M. Listeria monocytogenes ActA: A new function for a ‘classic’ virulence factor. Curr Opin Microbiol 2014; 17: 53–60. - PubMed
-
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol 2004; 4(10): 812–823. - PubMed
-
- North RJ, Dunn PL, Conlan JW. Murine listeriosis as a model of antimicrobial defense. Immunol Rev 1997; 158(1): 27–36. - PubMed
-
- Bouwer HG, Barry RA, Hinrichs DJ. Acquired immunity to an intracellular pathogen: Immunologic recognition of L. monocytogenes-infected cells. Immunol Rev 1997; 158: 137–146. - PubMed
-
- Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol 2006; 6(7): 520–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

