Wedge-shaped microfluidic chip for circulating tumor cells isolation and its clinical significance in gastric cancer
- PMID: 29792200
- PMCID: PMC5966930
- DOI: 10.1186/s12967-018-1521-8
Wedge-shaped microfluidic chip for circulating tumor cells isolation and its clinical significance in gastric cancer
Abstract
Background: Circulating tumor cells (CTCs) have great potential in both basic research and clinical application for the managements of cancer. However, the complicated fabrication processes and expensive materials of the existing CTCs isolation devices, to a large extent, limit their clinical translation and CTCs' clinical value. Therefore, it remains to be urgently needed to develop a new platform for achieving CTCs detection with low-cost, mass-producible but high performance.
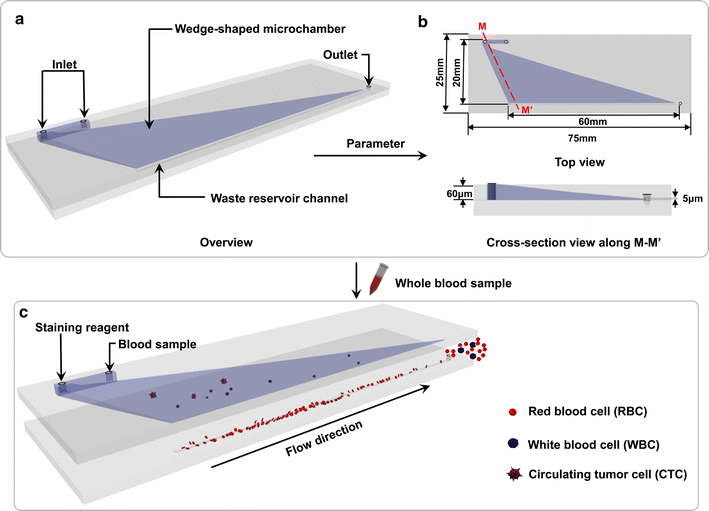
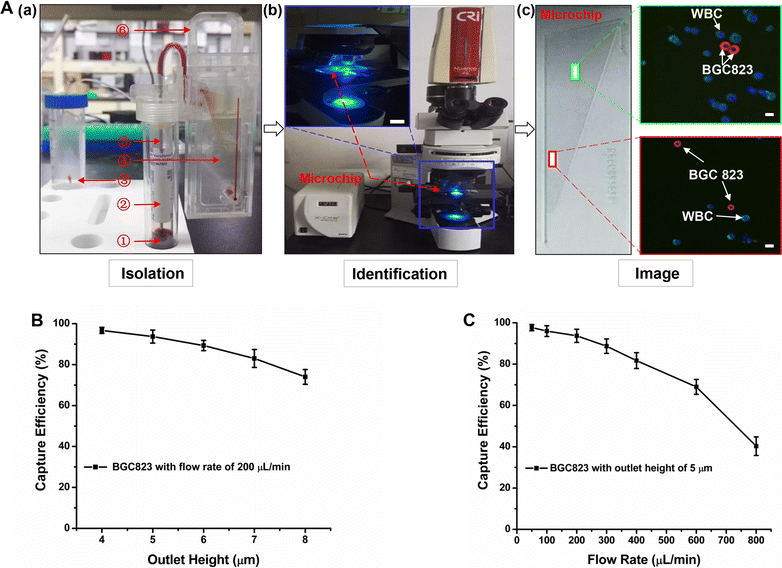
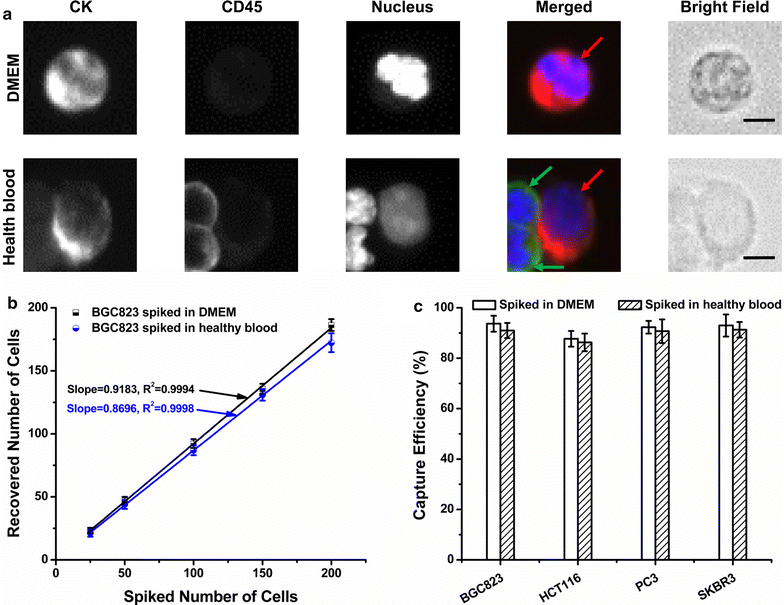
Methods: In the present study, we introduced a novel wedge-shaped microfluidic chip (named CTC-ΔChip) fabricated by two pieces of glass through wet etching and thermal bonding technique for CTCs isolation, which achieved CTCs enrichment by different size without cell surface expression markers and CTCs identification with three-color immunocytochemistry method (CK+/CD45-/Nucleus+). We validated the feasibility of CTC-ΔChip for detecting CTCs from different types of solid tumor. Furthermore, we applied the newly-developed platform to investigate the clinical significance of CTCs in gastric cancer (GC).
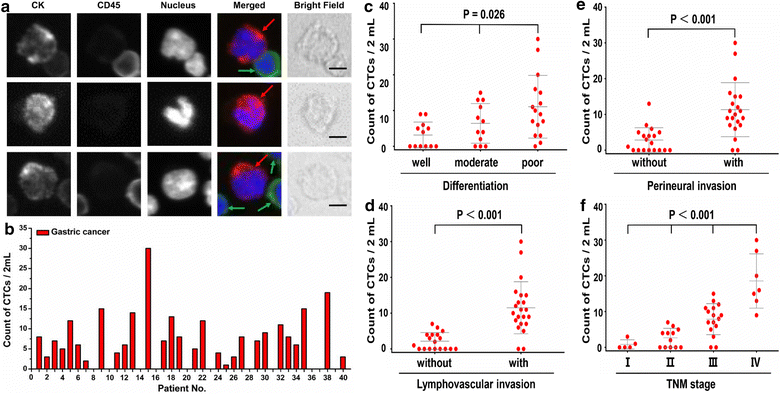
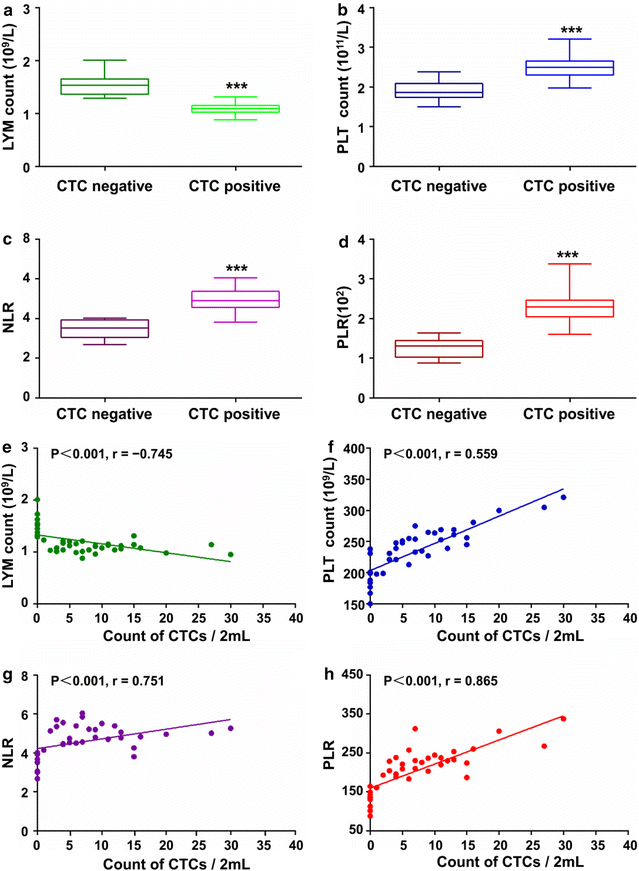
Results: Based on "label-free" characteristic, the capture efficiency of CTC-ΔChip can be as high as 93.7 ± 3.2% in DMEM and 91.0 ± 3.0% in whole blood sample under optimized conditions. Clinically, CTC-ΔChip exhibited the feasibility of detecting CTCs from different types of solid tumor, and it identified 7.30 ± 7.29 CTCs from 2 mL peripheral blood with a positive rate of 75% (30/40) in GC patients. Interestingly, we found that GC CTCs count was significantly correlated with multiple systemic inflammation indexes, including the lymphocyte count, platelet count, the level of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. In addition, we also found that both the positivity rate and CTCs count were significantly associated with multiple clinicopathology parameters.
Conclusions: Our novel CTC-ΔChip shows high performance for detecting CTCs from less volume of blood samples of cancer patients and important clinical significance in GC. Owing to the advantages of low-cost and mass-producible, CTC-ΔChip holds great potential of clinical application for cancer therapeutic guidance and prognostic monitoring in the future.
Keywords: Cell capture; Circulating tumor cells; Gastric cancer; Microfluidic; Wedge-shaped chip.
Figures
References
-
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. - DOI - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2009;20(7):1223–1229. doi: 10.1093/annonc/mdn786. - DOI - PubMed
-
- Normanno N, Rossi A, Morabito A, Signoriello S, Bevilacqua S, Di Maio M, Costanzo R, De Luca A, Montanino A, Gridelli C, et al. Prognostic value of circulating tumor cells’ reduction in patients with extensive small-cell lung cancer. Lung Cancer. 2014;85(2):314–319. doi: 10.1016/j.lungcan.2014.05.002. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- 81372358/National Natural Science Foundation of China/International
- 81527801/National Natural Science Foundation of China/International
- 2014CFA029/Natural Science Foundation of Hubei Province/International
- T201305/Colleges of Hubei Province Outstanding Youth Science and Technology Innovation Team/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

