Cerium dioxide nanoparticles exacerbate house dust mite induced type II airway inflammation
- PMID: 29792201
- PMCID: PMC5966909
- DOI: 10.1186/s12989-018-0261-5
Cerium dioxide nanoparticles exacerbate house dust mite induced type II airway inflammation
Abstract
Background: Nanomaterial inhalation represents a potential hazard for respiratory conditions such as asthma. Cerium dioxide nanoparticles (CeO2NPs) have the ability to modify disease outcome but have not been investigated for their effect on models of asthma and inflammatory lung disease. The aim of this study was to examine the impact of CeO2NPs in a house dust mite (HDM) induced murine model of asthma.
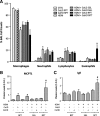
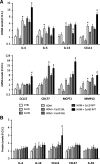
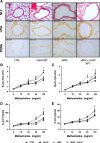
Results: Repeated intranasal instillation of CeO2NPs in the presence of HDM caused the induction of a type II inflammatory response, characterised by increased bronchoalveolar lavage eosinophils, mast cells, total plasma IgE and goblet cell metaplasia. This was accompanied by increases in IL-4, CCL11 and MCPT1 gene expression together with increases in the mucin and inflammatory regulators CLCA1 and SLC26A4. CLCA1 and SLC26A4 were also induced by CeO2NPs + HDM co-exposure in air liquid interface cultures of human primary bronchial epithelial cells. HDM induced airway hyperresponsiveness and airway remodelling in mice were not altered with CeO2NPs co-exposure. Repeated HMD instillations followed by a single exposure to CeO2NPs failed to produce changes in type II inflammatory endpoints but did result in alterations in the neutrophil marker CD177. Treatment of mice with CeO2NPs in the absence of HDM did not have any significant effects. RNA-SEQ was used to explore early effects 24 h after single treatment exposures. Changes in SAA3 expression paralleled increased neutrophil BAL levels, while no changes in eosinophil or lymphocyte levels were observed. HDM resulted in a strong induction of type I interferon and IRF3 dependent gene expression, which was inhibited with CeO2NPs co-exposure. Changes in the expression of genes including CCL20, CXCL10, NLRC5, IRF7 and CLEC10A suggest regulation of dendritic cells, macrophage functionality and IRF3 modulation as key early events in how CeO2NPs may guide pulmonary responses to HDM towards type II inflammation.
Conclusions: CeO2NPs were observed to modulate the murine pulmonary response to house dust mite allergen exposure towards a type II inflammatory environment. As this type of response is present within asthmatic endotypes this finding may have implications for how occupational or incidental exposure to CeO2NPs should be considered for those susceptible to disease.
Keywords: Asthma; Lung; Nanomaterial; Transcriptomics.
Conflict of interest statement
Ethics approval
All procedures were performed in accordance with the Animals (Scientific Procedures) Act 1986 which complies with EU Directive 2010/63/EU, including review and approval by the local Animal Welfare and Ethical Review Body.
Competing interests
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Fan L, Wang C, Chen M, Zhu B. Recent development of ceria-based (nano)composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J Power Sources. 2013;234:154–174. doi: 10.1016/j.jpowsour.2013.01.138. - DOI
-
- DeCoteau W, Heckman KL, Estevez AY, Reed KJ, Costanzo W, Sandford D, et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine. 2016;12(8):2311–2320. doi: 10.1016/j.nano.2016.06.009. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

