Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma
- PMID: 29792289
- PMCID: PMC6045511
- DOI: 10.5152/dir.2018.17528
Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma
Abstract
Purpose: We aimed to compare the clinical effectiveness of combination therapy of transarterial chemoembolization (TACE) and microwave ablation (MWA) with TACE monotherapy in BCLC stage B HCC patients with tumor size ≤7 cm and tumor number ≤5.
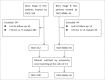
Methods: We retrospectively reviewed 150 BCLC stage B HCC patients who had received TACE monotherapy or TACE-MWA combination therapy in our hospital from March 2007 to April 2016. The patients were matched by propensity score at the ratio of 1:2 by optimal method. The median follow-up period was 16 months. The overall survival, tumor response and progression-free survival were compared between the two groups by Kaplan-Meier method and Log rank test.
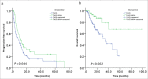
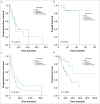
Results: Tumor response (complete or partial response or stable disease) rates at 6, 12, 18, 24 months were 55.5%, 37.3%, 21.3%, 15.8% for TACE group, and 74%, 47.8%, 35%, 31.8% for TACE-MWA group, respectively. The survival rates at 1, 3, 5 years were 77.5%, 42.1%, 21% for TACE group and 93.1%, 79%, 67.7% for TACE-MWA group, respectively. Compared with TACE group, the TACE-MWA group had significantly improved progression-free survival (P = 0.044) and overall survival (P = 0.002).
Conclusion: TACE-MWA combination therapy has better clinical effectiveness than TACE monotherapy in BCLC stage B patients with tumor size ≤7 cm and tumor number ≤5.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

