PEDF regulates plasticity of a novel lipid-MTOC axis in prostate cancer-associated fibroblasts
- PMID: 29792311
- PMCID: PMC6051346
- DOI: 10.1242/jcs.213579
PEDF regulates plasticity of a novel lipid-MTOC axis in prostate cancer-associated fibroblasts
Abstract
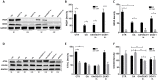
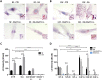
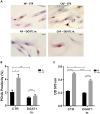
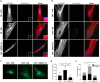
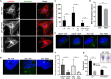
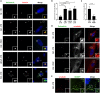
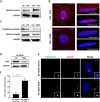
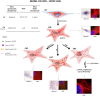
Prostate tumors make metabolic adaptations to ensure adequate energy and amplify cell cycle regulators, such as centrosomes, to sustain their proliferative capacity. It is not known whether cancer-associated fibroblasts (CAFs) undergo metabolic re-programming. We postulated that CAFs augment lipid storage and amplify centrosomal or non-centrosomal microtubule-organizing centers (MTOCs) through a pigment epithelium-derived factor (PEDF)-dependent lipid-MTOC signaling axis. Primary human normal prostate fibroblasts (NFs) and CAFs were evaluated for lipid content, triacylglycerol-regulating proteins, MTOC number and distribution. CAFs were found to store more neutral lipids than NFs. Adipose triglyceride lipase (ATGL) and PEDF were strongly expressed in NFs, whereas CAFs had minimal to undetectable levels of PEDF or ATGL protein. At baseline, CAFs demonstrated MTOC amplification when compared to 1-2 perinuclear MTOCs consistently observed in NFs. Treatment with PEDF or blockade of lipogenesis suppressed lipid content and MTOC number. In summary, our data support that CAFs have acquired a tumor-like phenotype by re-programming lipid metabolism and amplifying MTOCs. Normalization of MTOCs by restoring PEDF or by blocking lipogenesis highlights a previously unrecognized plasticity in centrosomes, which is regulated through a new lipid-MTOC axis.This article has an associated First Person interview with the first author of the paper.
Keywords: ATGL (PNPLA2); CAF; Centrosome; MTOC; PEDF (SERPINF); β-catenin.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
DGAT1 Inhibitor Suppresses Prostate Tumor Growth and Migration by Regulating Intracellular Lipids and Non-Centrosomal MTOC Protein GM130.Sci Rep. 2019 Feb 28;9(1):3035. doi: 10.1038/s41598-019-39537-z. Sci Rep. 2019. PMID: 30816200 Free PMC article.
-
PEDF promotes nuclear degradation of ATGL through COP1.Biochem Biophys Res Commun. 2019 May 14;512(4):806-811. doi: 10.1016/j.bbrc.2019.03.111. Epub 2019 Mar 27. Biochem Biophys Res Commun. 2019. PMID: 30926171
-
Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL).J Hepatol. 2008 Mar;48(3):471-8. doi: 10.1016/j.jhep.2007.10.012. Epub 2007 Dec 26. J Hepatol. 2008. PMID: 18191271
-
Cancer cell apoptotic pathways mediated by PEDF: prospects for therapy.Trends Mol Med. 2009 Oct;15(10):461-7. doi: 10.1016/j.molmed.2009.08.003. Epub 2009 Sep 25. Trends Mol Med. 2009. PMID: 19783213 Review.
-
Pigment epithelium-derived factor in lipid metabolic disorders.Biomed J. 2018 Apr;41(2):102-108. doi: 10.1016/j.bj.2018.02.004. Biomed J. 2018. PMID: 29866598 Free PMC article. Review.
Cited by
-
Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics.J Hematol Oncol. 2023 Sep 12;16(1):103. doi: 10.1186/s13045-023-01498-2. J Hematol Oncol. 2023. PMID: 37700339 Free PMC article. Review.
-
Lipid Metabolism in Tumor-Associated Fibroblasts.Adv Exp Med Biol. 2021;1316:117-131. doi: 10.1007/978-981-33-6785-2_8. Adv Exp Med Biol. 2021. PMID: 33740247 Review.
-
Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment.Oncogene. 2019 Jun;38(25):4887-4901. doi: 10.1038/s41388-019-0765-y. Epub 2019 Feb 28. Oncogene. 2019. PMID: 30816343 Review.
-
Fibroblasts as Modulators of Local and Systemic Cancer Metabolism.Cancers (Basel). 2019 May 3;11(5):619. doi: 10.3390/cancers11050619. Cancers (Basel). 2019. PMID: 31058816 Free PMC article. Review.
-
PEDF and Its Role in Metabolic Disease, Angiogenesis, Cardiovascular Disease, and Diabetes.Biomedicines. 2025 Jul 21;13(7):1780. doi: 10.3390/biomedicines13071780. Biomedicines. 2025. PMID: 40722853 Free PMC article. Review.
References
-
- Banerjee S., Zare R. N., Tibshirani R. J., Kunder C. A., Nolley R., Fan R., Brooks J. D. and Sonn G. A. (2017). Diagnosis of prostate cancer by desorption electrospray ionization mass spectrometric imaging of small metabolites and lipids. Proc. Natl. Acad. Sci. USA 114, 3334-3339. 10.1073/pnas.1700677114 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

