Gastroretentive raft liquid delivery system as a new approach to release extension for carrier-mediated drug
- PMID: 29792353
- PMCID: PMC6058684
- DOI: 10.1080/10717544.2018.1474969
Gastroretentive raft liquid delivery system as a new approach to release extension for carrier-mediated drug
Abstract
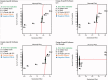
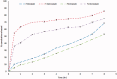
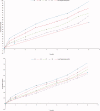
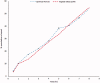
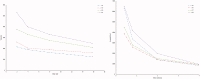
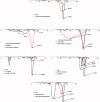
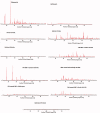
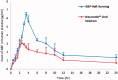
Gabapentin (GBP), an antiepileptic and anti-neuropathic agent, suffers from short half-life (5-7 h), has narrow absorption window, and is absorbed via carrier-mediated mechanism resulting in frequent dosing, poor compliance, and poor bioavailability (<60%). Moreover, GBP is a freely water-soluble drug, thus it is considered a challenging candidate to be formulated as extended release dosage form. In this study, raft forming systems were investigated as a potential drug delivery system for prolonging gastric residence time of GBP. A 23 full factorial design was adopted to study the effect of formulation variables (% gellan gum, % GMO, and % LM-pectin 101), on the percent of GBP released at different time intervals (1, 5, and 8 h) as well as the gel strength, and thus was achieved an optimized formula with zero-order release profile suitable for once-daily administration. In vivo assessment was performed in rats to evaluate gastric residence of the gel formed. In addition, the oral bioavailability of GBP relative to commercially available Neurontin® immediate release oral solution was also investigated. Significant increase was observed for Cmax, AUC(0-t), and AUC(0-∞). The increase in relative bioavailability of GBP from the optimized formula was 1.7 folds.
Keywords: GMO; Gabapentin; LM-pectin 101; gellan gum; raft forming systems.
Figures






Similar articles
-
Development and optimization of gastroretentive mucoadhesive microspheres of gabapentin by Box-Behnken design.Artif Cells Nanomed Biotechnol. 2014 Jun;42(3):167-77. doi: 10.3109/21691401.2013.800081. Epub 2013 Jun 13. Artif Cells Nanomed Biotechnol. 2014. PMID: 23763514
-
Pharmacokinetics of immediate release, extended release, and gastric retentive gabapentin formulations in healthy adults .Int J Clin Pharmacol Ther. 2018 May;56(5):231-238. doi: 10.5414/CP203166. Int J Clin Pharmacol Ther. 2018. PMID: 29633699 Free PMC article. Clinical Trial.
-
Utilization of ionotropic gelation technique for bioavailability enhancement of cinnarizine: in-vitro optimization and in-vivo performance in human.Drug Deliv. 2016 Oct;23(8):2736-2746. doi: 10.3109/10717544.2015.1064187. Epub 2015 Jul 13. Drug Deliv. 2016. PMID: 26165421
-
Clinical development of a once-daily gastroretentive formulation of gabapentin for treatment of postherpetic neuralgia: an overview.Expert Opin Drug Deliv. 2012 Sep;9(9):1147-60. doi: 10.1517/17425247.2012.709231. Epub 2012 Jul 18. Expert Opin Drug Deliv. 2012. PMID: 22809245 Review.
-
The intestinal absorption mechanism of gabapentin makes it appropriate for gastroretentive delivery.Curr Clin Pharmacol. 2013 Feb 1;8(1):67-72. Curr Clin Pharmacol. 2013. PMID: 22946876 Review.
Cited by
-
In-Depth Study into Polymeric Materials in Low-Density Gastroretentive Formulations.Pharmaceutics. 2020 Jul 7;12(7):636. doi: 10.3390/pharmaceutics12070636. Pharmaceutics. 2020. PMID: 32645909 Free PMC article. Review.
-
Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications.Pharmaceutics. 2021 Sep 30;13(10):1591. doi: 10.3390/pharmaceutics13101591. Pharmaceutics. 2021. PMID: 34683884 Free PMC article. Review.
-
Merging konjac glucomannan with other copolymeric hydrogels as a cutting-edge liquid raft system for dual delivery of etoricoxib and famotidine.Drug Deliv. 2023 Dec;30(1):2189630. doi: 10.1080/10717544.2023.2189630. Drug Deliv. 2023. PMID: 36927148 Free PMC article.
-
Mechanically Robust Gastroretentive Drug-Delivery Systems Capable of Controlling Dissolution Behaviors of Coground β-Lapachone.Pharmaceutics. 2019 Jun 10;11(6):271. doi: 10.3390/pharmaceutics11060271. Pharmaceutics. 2019. PMID: 31185692 Free PMC article.
-
Maximizing the Use of Ivermectin Transethosomal Cream in the Treatment of Scabies.Pharmaceutics. 2024 Aug 1;16(8):1026. doi: 10.3390/pharmaceutics16081026. Pharmaceutics. 2024. PMID: 39204371 Free PMC article.
References
-
- Abou Youssef NA, Kassem AA, El-Massik MA, Boraie NA. (2015). Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int J Pharm 486:297–305. - PubMed
-
- Abouelatta SM, Aboelwafa AA, Khalil RM, ElGazayerly ON. (2015). Floating lipid beads for the improvement of bioavailability of poorly soluble basic drugs: in-vitro optimization and in-vivo performance in humans. Eur J Pharm Biopharm 89:82–92. - PubMed
-
- Abouelatta SM, Aboelwafa AA, Khalil RM, El-Gazayerly ON. (2016). Utilization of ionotropic gelation technique for bioavailability enhancement of cinnarizine: in-vitro optimization and in-vivo performance in human. Drug Deliv 23:2736–46. - PubMed
-
- Ahuja M, Singh S, Kumar A. (2013). Evaluation of carboxymethyl gellan gum as a mucoadhesive polymer. Int J Biol Macromol 53:114–21. - PubMed
-
- Basha M, Abd El-Alim SH, Shamma RN, Awad GE. (2013). Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J Liposome Res 23:203–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
